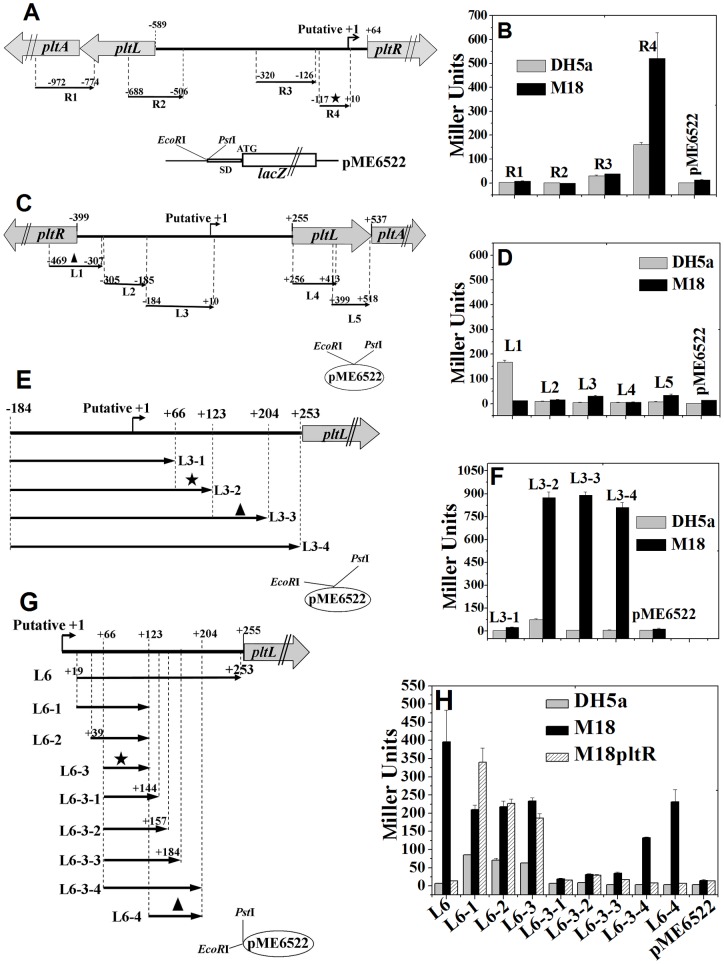
Figure 1. Detection of promoter activity in the intergenic region between divergently transcribed pltR and pltLABCDEFG genes using the lacZ reporter gene.
(A and B) Promoter detection in the pltR direction. (A) Four fragments, R1 to R4, containing five putative promoters in the pltR direction (predicted by the NNPP promoter online prediction with a score cutoff 0.80) were fused with the promoterless lacZ gene on the plasmid pME6522. The R3 fragment includes two putative promoters. The putative +1 site indicates the putative TSS of the fifth predicted promoter. (B) β-Galactosidase expression (Miller Units) from above these lacZ fusion plasmids was assayed in both E. coli DH5α and P. aeruginosa M18 after 15 h of growth in KMB. The R4 fragment was detected to include a true promoter (shown by an asterisk). (C and D) Promoter detection in the pltLABCDEFG operon direction. (C) Five fragments, L1 to L5, which contain five putative promoters in the pltL direction, were respectively fused into the upstream of the promoterless lacZ gene on pME6522. Putative +1 indicates the putative TSS of the third predicted promoter. (D) β-Galactosidase expression from the above lacZ fusion recombined plasmids was measured in both DH5α and M18. The L1 fragment (marked with a triangle) exhibited a certain degree of promoter activity in DH5α, but not in M18. The other four fragments, L2 to L5, did not show any evident promoter activity. (E and F) Detection of promoter or its elements by prolongation analysis within the DNA fragment spanning from +10 to the pltL ATG, which was not predicted to carry promoters. (E) Four prolonging fragments based on L3, namely, L3–1 to L3–4, were respectively fused with the lacZ gene in pME6522. (F) The resulting lacZ fusion plasmids were assayed for β-galactosidase expressions in both DH5α and M18. The region covering +66 to +124 bp (relative to the putative +1 site) contained either a stronger promoter or its elements (marked with an asterisk). The L3–3–lacZ fusion expression significantly decreased, compared with that in the L3–2–lacZ fusion expression in E. coli DH5α, which implies that the promoter located at +66 to +124bp is not likely pltLp activated by PltR. Moreover, the region from +124 to +205 bp (marked with a triangle) possibly contains another promoter under the transcriptional activation of PltR. (G and H) Distinguishing pltLp activated by PltR from other promoters by deletion analysis. (G) Four fragments (L6 and its shortened derivative fragments, namely, L6–1 to L6–3), four continuously prolonged fragments based on L6–3 (L6-3-1 to L6-3-4), and the L6–4 fragment, were respectively cloned into pME6522. The L6 fragment was not predicted (score cutoff 0.60) to contain putative promoter regions. (H) The β-galactosidase expression from these recombinant lacZ reporter plasmids was analyzed in E. coli DH5α, P. aeruginosa M18, and the pltR mutant M18pltR. An intact promoter contained in the region from +66 to +124 (marked with an asterisk) was not controlled by PltR. The pltLp activated by PltR was located from +124 to +205 bp (marked with a triangle), relative to the putative +1 site.