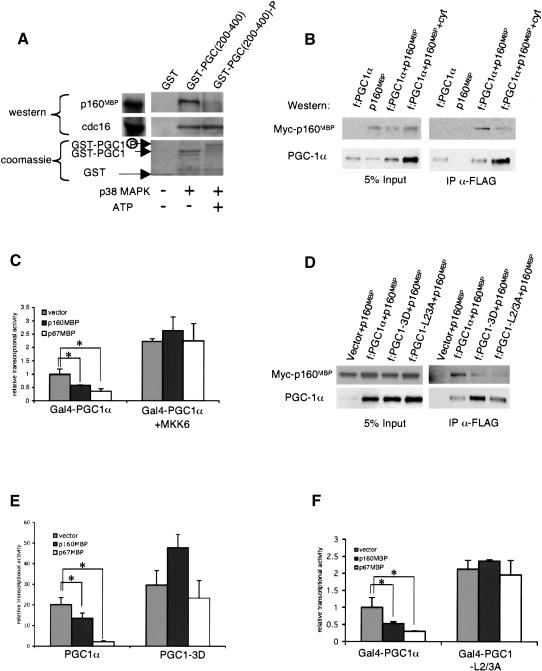
Figure 3.
p160MBP's interaction with PGC-1α is modulated by p38 MAPK phosphorylation. (A) GST-PGC1(200-400) was phosphorylated in vitro by activated p38 MAPK in the presence of ATP [GST-PGC1(200-400)-P] or mock phosphorylated in the absence of ATP [GST-PGC1(200-400)]. The GST proteins were then incubated with C2C12 nuclear extracts and interacting proteins were visualized by Western blot by using antibodies against p160MBP or against cdc16 (Santa Cruz Biotechnology). (B) Flag-tagged PGC-1α (f:PGC1α) was cotransfected with myc-tagged p160MBP (myc-p160MBP) in BOSC cells and after 24 h treated with the cytokines IL-1α, IL-1β, and TNFα overnight in DMEM + 0.5% BSA. Whole-cell extracts were immunoprecipitated with M2 anti-Flag antibodies (Sigma) and Western blots were conducted with antibodies against myc or PGC-1α (Santa Cruz Biotechnology). The left panel is a Western blot of 5% of the whole-cell extract before immunoprecipitation and the right panel is a Western blot of the immunoprecipitated proteins. (C) Gal4-PGC1α was cotransfected in C2C12 cells with a UAS-luciferase reporter and a vector expressing MKK6, the upstream activator of p38 MAPK. Medium was changed to DMEM + 0.5% BSA after 24 h, and luciferase activity was measured after 48 h. (Asterisks) P < 0.05, paired t-test. (D) Myc-p160MBP was cotransfected with f:PGC1α, f:PGC1α with the phosphorylation sites mutated to aspartic acid (f:PGC1-3D), or f:PGC1α with selected leucines at L2 and L3 mutated to alanine (f:PGC1-L2/3A). Extracts were immunoprecipitated with anti-Flag antibodies as described earlier. (E) PGC-1α or PGC1-3D were cotransfected with PPARγ, RXR, and DR-1 luciferase reporter in HIB1B cells. Cells were harvested after 24 h and fold activation over PPARγ was graphed. (Asterisks) P < 0.05, paired t-test. (F) Gal4-PGC1 or Gal4-PGC1-L2/3A were cotransfected with a UAS-TATA-luciferase reporter in C2C12 cells, and luciferase activity was measured after 24 h. (Asterisks) P < 0.05, paired t-test.