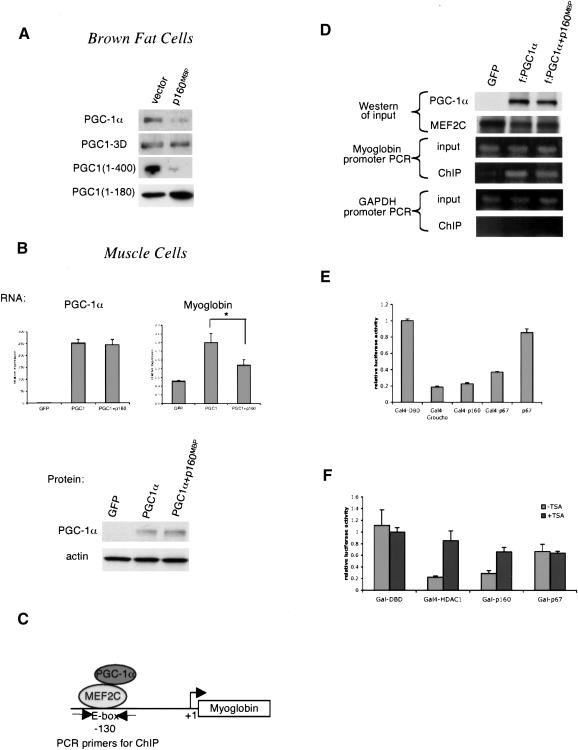
Figure 5.
p160MBP does not alter PGC-1α protein level or recruitment to DNA. (A) HIB1B brown fat cells were cotransfected with p160MBP and various versions of PGC-1α. Cell extracts were Western blotted for PGC-1α. (B) C2C12 myotubes were coinfected with adenoviral MEF2C, PGC-1α, and p160MBP and harvested for RNA and protein. RNA was subjected to real-time PCR analysis to determine the amounts of PGC-1α and myoglobin transcripts. Protein was blotted with antibodies against PGC-1α or the loading control actin. (Asterisk) P < 0.05, paired t-test. (C) Diagram of PGC-1α interaction with MEF2C on the myoglobin promoter; sites for PCR primers for chromatin immunoprecipitation assays are indicated. (D) C2C12 myotubes were coinfected with adenoviral MEF2C, Flag-tagged PGC-1α, and p160MBP. Chromatin immunoprecipitations were carried out using M2 anti-Flag antibodies. The input and immunoprecipitated DNA were used as templates for PCR with primers flanking the MEF2C site at -130 on the myoglobin promoter. Primers amplifying a region of the GAPDH gene were used as a negative control. (E) Gal4 fusion protein constructs were transfected into C2C12 myoblasts with a UAS-tk-luciferase reporter. The cells were harvested after 24 h and luciferase activity was measured. (F) Similar to E, except 100 μg/mL trichostatin A (TSA) was added after 12 h.