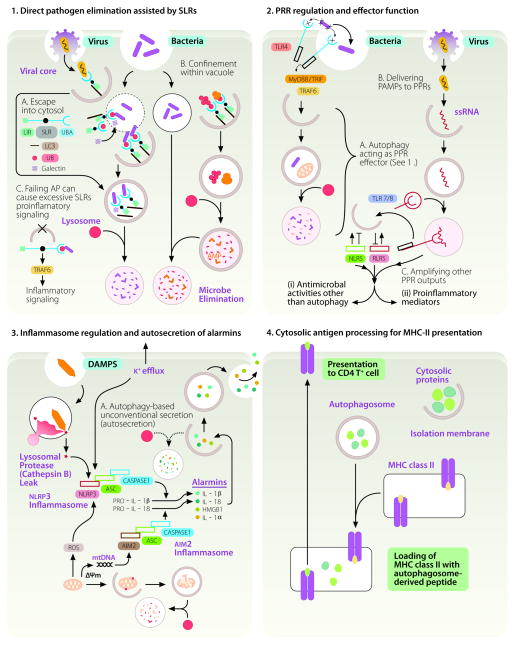
FIGURE 1.
The four principal manifestations of immunological autophagy. 1. Direct pathogen elimination assisted by SLRs and DAMP receptors. (A) Invading microbes either escaping the endosomes or phagosome (thin outline) or remaining in phagosomes that can be partially permeabilized (dotted outline) are captured by galectins and sequestosome 1/p62-like receptors (SLR) that recognize tags such as ubiquitin (small red circles) or diacylglicerol and β-galactoside (not shown) on damaged host membranes. The captured microbes or co-captured with the earmarked membranes are delivered into autophagic organelles (thick outline) starting with phagophores (crescents) progressing through autophagosomes (full white circles) and ending in degradative autolysosomes (full pink circles). SLRs posses LC3 interacting region (LIR), phosphorylation sites (black dot, arbitrarily positioned), and tag recognition domain (UBA, depicted for p62). Galectins (hatched square), considered to be DAMP receptors, have carbohydrate recognition domains (not shown) that recognize sugars on glycans exposed on the endofacial lumenal membrane leaflet of permeabilized organelles. (B) Alternatively, autophagy can sequester cytosolic proteins such as ubiquitin and ribosomal proteins (pear shaped tan-colored objects, ribosomes) and digest them into antimicrobial peptides (AMPs) that can be delivered to pathogens confined in phagosomes. (C) SLRs can engage in pro-inflammatory signaling e.g. via TRAF6 (shown) or atypical PKC (not shown) or promote cell death by activating caspase-8 through aggregation (not shown). AP, autophagy. 2. PRR regulation and effector functions. A. Autophagy can be activated downstream of TLR signaling upon recognition of PAMPs (x-like objects). B. As a topological inverter device, autophagy can deliver cytosolic PAMPs to the lumen of endomembranous organelles where they can interact with the receptor portions of TLRs. Known functional interactions with NLRs and RLRs are summarized by positive (arrows) and negative (lines symbolizing inhibition) effects. 3. Inflammasome regulation and secretion of alarmins. Autophagy plays a dual role in controlling inflammasome output: it suppresses basal levels of inflammasome activation but also assists IL-1βand IL-18 release from the cells via an autophagy-dependent unconventional secretory pathway (A; autosecretion). Inflammasomes, heteromeric protein assemblages (consisting of ASC, caspase 1, and NLRP3 or AIM2) act as platforms for activation in response to K+ efflux or presence and action of DAMPs (silica, crystal like object; ROS, reactive oxygen species; mtDNA, mitochondrial DNA). ROS and mtDNA can be released as endogenous DAMPs by damaged mitochondria if they are not continuously removed by autophagy. This results in caspase-1 activation and proteolytic processing of pro-forms of proinflammatory cytokines (IL-1β). Whereas autophagy lowers the sources of endogenous DAMPs by disposing of depolarized (ΔΨm) or leaky mitochondria (sources of ROS and mtDNA), autophagy also enables secretion of the cytosolic IL-1β(and other alarmins such as HMGB1) during the very early stages of physiological inflammasome activation in response to exogenous DAMP sources (microbial or sterile). Autosecretion (autophagy-based unconventional secretion; see Fig. 2 and text for explanations) enables extracellular release of the cytosolic proteins such as IL-1β and HMGB1 per the illustrated process controlled by Atg factors and GRASP (see Fig. 2). Autosecretion occurs early in the process of stimulation and is swamped pre- or shortly post-stimulation by the anti-inflammatory effects of autophagy. The latter keep the tonic levels of inflammasome activation low and bring them back to the resting levels following stimulation. 4. Cytosolic antigen processing for antigen presentation. Autophagy assists as a topological inversion device in delivery of cytosolic (and nuclear) proteins to MHC II processing and presentation compartments. Explanations in the text include relationships to selection of naïve T cell repertoires and citrullination of antigens.