Abstract
Attention-Deficit/Hyperactivity Disorder (ADHD) is associated with deficits in fluid reasoning, which may be related to self-regulation of cognition and behavior, and requires intact attention, working memory, and inhibition skills. No functional magnetic resonance imaging (fMRI) studies have directly examined fluid reasoning in ADHD which is surprising given that studies demonstrate a consistent network of brain regions involved in fluid reasoning that are also implicated in the pathogenesis of ADHD. Twenty-two right-handed, non-medicated children (12 ADHD, 10 controls) ages 8–12 years completed a fluid reasoning task during which fMRI data were collected. The primary comparison of interest was activation during the fluid reasoning compared to the control condition. Behavioral data showed that children with ADHD tended to be less accurate with faster reaction times in the fluid reasoning condition compared to controls, and were significantly less accurate in the control condition. Controls activated more than participants with ADHD in the right intraparietal sulcus and the left lateral cerebellum in the fluid reasoning condition. Results showed hypoactivation in ADHD in regions critical for fluid reasoning. These results add to the literature suggesting a role for parietal and cerebellar regions in cognition and ADHD.
Keywords: ADHD, fluid reasoning, relational reasoning, cerebellum, fMRI
1. Introduction
Attention-Deficit/Hyperactivity Disorder (ADHD) is a behaviorally defined disorder affecting approximately 3–5% of school-aged children (American Psychological Association, 1994). ADHD is characterized by problems with inattention, impulsivity, and hyperactivity causing significant impairment in multiple settings. Accumulating evidence indicates that ADHD is associated with core deficits in executive functioning (Barkley, 1997; Nigg et al., 2004; Pennington and Ozonoff, 1996; Sergeant et al., 2002; Shallice et al., 2002). Executive functioning encompasses a diverse set of cognitive processes including higher order planning, working memory, inhibition, and set shifting. Several programs of research have contributed to the large literature on the executive functioning deficits associated with ADHD, including state-regulation deficits (Douglas, 1972; Douglas, 1988; Leth-Steenson et al., 2000), energetic deficits (Sergeant et al., 1999; Sergeant, 2000), the behavioral inhibition deficit model (Barkley, 1997), impaired controlled and automatic processing (Carte et al., 1996; Nigg, 2001; Nigg et al., 2004), and inefficiencies in attentional networks (Swanson et al., 1998). Although there is broad agreement that many ADHD children show some form of impairment in executive functioning, studies have not been consistent in associating specific executive functioning domains with ADHD (Barkley, 1997; Marks et al., 2005; Nigg et al., 2004; Pennington and Ozonoff, 1996; Sergeant et al., 2002). For example, studies have associated ADHD with impairment in response inhibition as indexed by errors of commission on a continuous performance test; (Barkley, 1997; Nigg et al., 1996; Pasini et al., 2007), speed of inhibition to an auditory stop signal (Schachar and Logan, 1990), and working memory (Barkley, 1997; Berlin et al., 2004; Kuntsi et al., 2001). Other studies have suggested that ADHD children have slower and more variable response speed as a common feature across all cognitive tasks (Castellanos and Tannock, 2002; Kuntsi et al., 2001).
Fluid reasoning, also known as analogical or relational reasoning, is the ability to manipulate representations among stimuli in order to reason, plan, and problem solve using attentional, working memory, and cognitive perceptual skills (Cho et al., 2010; Morrison et al., 2004). It involves relational integration, inhibitory control, and resolution of interference and is considered a core component of fluid intelligence (Morrison et al., 2004). In fact, fluid reasoning appears critical for all tasks identified with executive functioning (Cho et al., 2010) and may comprise an executive function resource that influences self-regulation of cognition and behavior (Blair, 2006).
The fact that working memory, attention, inhibition, and interference resolution are key to successful fluid reasoning (Cho et al., 2010; Morrison et al., 2004; Waltz et al., 1999), and that these are all areas of deficit in ADHD (Nigg et al., 1999; Nigg et al., 2002; Nigg et al., 2004; Willcutt et al., 2005), suggests that we might anticipate behavioral differences from participants with ADHD on tasks assessing fluid reasoning, e.g., Matrix Reasoning on the Wechsler Scales (Wechsler, 2002; Wechsler, 2003; Wechsler, 2004) and the Raven’s Progressive Matrices task (Raven, 1965). And, in fact, it has been demonstrated that individuals with ADHD perform more poorly on such tasks which are considered analogs of non-verbal IQ (da Rocha et al., 2008; Garcia-Sanchez et al., 1997; Klingberg et al., 2002; Liu and Wang, 2002; Ma et al., 2011; Semrud-Clikeman et al., 2010) although few studies were found reporting on fluid reasoning in ADHD, and not all studies report group differences on this measure, e.g., (Rubia et al., 2009).
Interestingly, cognitive training interventions, targeting attention, working memory, and/or inhibition, for participants with ADHD (Kerns, 1999; Klingberg et al., 2002; Tamm et al., 2010) and for typically developing children (Rueda et al., 2005) have shown a positive impact on fluid reasoning even though the construct was not specifically targeted by the training intervention. This improvement is unlikely due to practice effects because the construct is typically stable on test-retest intervals (Nyden et al., 2001; Wechsler, 2004). Thus, the cognitive training literature further supports a relationship between attention, inhibition, working memory, and fluid reasoning.
The network of brain regions involved in fluid reasoning is comprised of the frontopolar region, middle and inferior frontal gyri, and parietal and occipital regions (Cho et al., 2010; Christoff et al., 2001; Eslinger et al., 2008; Geake and Hansen, 2005; Goel and Dolan, 2001; Gray et al., 2003; Knauff et al., 2002; Kroger et al., 2002; Lee et al., 2006; Prabhakaran et al., 1997; Ruff et al., 2003; Watson and Chatterjee, 2012; Wendelken et al., 2008), with a major role for the dorsolateral prefrontal cortex for more complex relations (Christoff et al., 2001; Kroger et al., 2002). Some studies also report a role for the cerebellum in fluid reasoning (Kalbfleisch et al., 2007). The same regions involved in fluid reasoning are also implicated by both functional and structural imaging studies in the pathogenesis of ADHD (Dickstein et al., 2006; Paloyelis et al., 2007). However, no studies were found specifically examining brain activation differences in individuals with ADHD on fluid reasoning tasks. Rubia and colleagues reported findings comparing children with ADHD to those with conduct disorder on a task requiring cognitive flexibility or switching (Rubia et al., 2010), a correlate of fluid reasoning. The data revealed that only individuals with ADHD demonstrated a switch reaction time cost and that this was associated with hypoactivation in the right inferior prefrontal region. Hypoactivation in frontal regions (anterior cingulate) was also observed on a response inhibition task for individuals with ADHD compared to controls which was theorized to be related to deficits in task switching (Tamm et al., 2004). Thus, indirect imaging evidence from tasks involving cognitive flexibility and task switching suggests that individuals with ADHD may under-activate brain regions associated with fluid reasoning.
The current study examined brain activation differences among ADHD participants compared to typically developing controls during a fluid reasoning task (Eslinger et al., 2008) in order to elucidate a possible neurological basis for their executive functioning deficits. We argue that it may be important to examine fluid reasoning in ADHD given its relationship to known neuropsychological and behavioral deficits in ADHD. Fluid reasoning has also been linked to social competence in children with ADHD (Schafer and Semrud-Clikeman, 2008), and low fluid reasoning in children predicts poorer academic outcomes (Lynn et al., 2007). We hypothesized that individuals with ADHD would perform more poorly on the fluid reasoning task than controls. We hypothesized that children with ADHD compared to controls would show hypoactivation in the regions previously shown to be involved in fluid reasoning, i.e., frontopolar region, middle and inferior frontal gyri, and parietal and occipital regions.
2. Results
Demographic Comparisons
The two groups did not differ significantly on age, gender, ethnicity, or WISC-IV matrix reasoning performance (Table 1). As expected, the ADHD group had significantly greater scores than controls on the SNAP-IV ADHD rating scale.
Table 1.
Demographic Characteristics
| ADHD n=12 | Control n=10 | Statistical Comparison | |
|---|---|---|---|
| Mean age (SD) | 9.0 years (1.3) | 10.0 years (1.4) | t (21) = −1.8, p = .09 |
| % Male | 54% | 80% | χ2 (1) = 1.7, p = .19 |
| Ethnicity | 54% Caucasian | 90% Caucasian | χ2 (3) = 4.9, p = .18 |
| 23% Hispanic | 10% African American | ||
| 15% Asian | |||
| 8% Other | |||
| ADHD Subtype | 23% Combined Type | Not applicable | Not applicable |
| 69% Inattentive Type | |||
| 8% Not otherwise specified | |||
| Comorbid Diagnosis | Oppositional Defiant Disorder (n=1) | Not applicable | Not applicable |
| Enuresis (n=1) | |||
| Separation Anxiety Disorder (n=1) | |||
| SNAP-IV Score | 1.7 (.39) | .24 (.20) | t (21) = 10.8, p < .001 |
| Matrix Reasoning | 11.7 (2.3) | 11.0 (2.7) | t (21) = .67, p = .51 |
Behavioral Comparisons of Task Performance While Inside the Scanner
While the groups did not significantly differ on fluid reasoning accuracy, the participants with ADHD tended to be less accurate than controls (73% accuracy versus 79% accuracy; effect size = .46). During the control condition, the ADHD group was significantly less accurate than controls (effect size 1.0) although the magnitude of this difference was less (97% accuracy versus 99% accuracy). Reaction time comparisons revealed the ADHD group tended to respond more quickly than controls in the fluid reasoning condition (effect size = .63). During the control condition, the ADHD and control groups performed similarly in terms of reaction time. Means and standard deviations for behavioral performance in the scanner are reported in Table 2.
Table 2.
Behavioral Performance in the Scanner
| ADHD n=12 | Control n=10 | Statistical Comparison | |
|---|---|---|---|
| Fluid reasoning accuracy | .73 (.11) | .79 (.15) | t (21) = −1.09, p=.14; d=.46 |
| Control accuracy | .97 (.02) | .99 (.02) | t (21) = −1.67, p=.05; d=1.0 |
| Fluid reasoning RT | 2078.0 (247.8) | 2219.1 (201.0) | t (21) = −1.47, p=.08; d=.63 |
| Control RT | 1180.3 (230.2) | 1162.7 (257.3) | t (21) = 0.17, p=.43; d=.07 |
RT = reaction time; d = effect size
Imaging Analyses
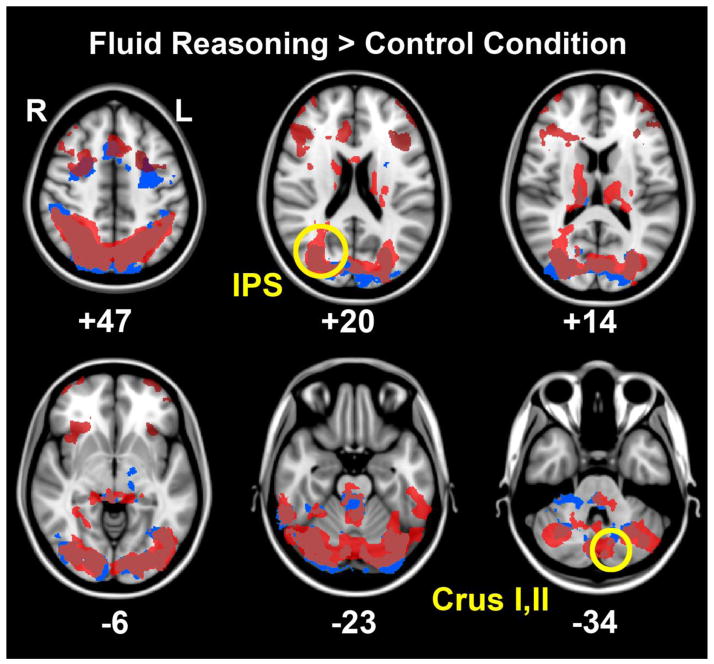
Both ADHD and controls activated a similar network of cortical regions to complete the fluid reasoning task. As shown in figure 1, these areas included frontal regions bilaterally (e.g., dorsolateral/rostrolateral prefrontal cortex and anterior cingulate), a posterior temporal region bilaterally (e.g., inferior temporal), a parietal region bilaterally (e.g., inferior parietal sulcus), occipital regions bilaterally (e.g., pericalcarine and lateral occipital), and cerebellar regions bilaterally (e.g. Crus I, II). Significant group effects (control>ADHD) were limited to the right intraparietal sulcus and the left Crus I, II cerebellar regions (Table 3, Figures 1 & 2). Participants with ADHD did not exhibit greater activation than controls in any region in the fluid reasoning condition compared to the control condition (Table 3). Significant main effects of age (greater BOLD signal with increasing age) were evident in the right lingual gyrus, the right anterior cerebellum, the left lateral occipital, and the lateral aspect of the left lateral cerebellum (e.g., Crus II). However, there were no significant interactions between group and age. Furthermore, none of the regions affected by age spatially overlapped with regions with significant group effects.
Figure 1.
The average ADHD group response (blue) and the average control group response (red) during the fluid reasoning > control condition are displayed at six different levels in the transverse plane to illustrate the distributed network involved in this task and the common regions activated by both groups. Highlighted by yellow circles are significant group differences (control>ADHD) in BOLD signal activations (cluster P threshold <0.05; Z threshold >2.3) evident in the right intraparietal sulcus and the left lateral cerebellum (Crus I and II).
Table 3.
Areas in which there were group differences on the fluid reasoning > control condition comparison with age covaried.
| Contrast | Region | Cluster size (voxels) | MNI Coordinates | Cluster significance (p) | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| ADHD > Control | None | |||||
| Control > ADHD | Intraparietal sulcus (right hemisphere) | 5852 | 33 | −73 | 14 | .00345 |
| Age | Crus I/II lateral (left hemisphere) | 5425 | −8 | −78 | −31 | .00569 |
| Anterior cerebellum (right hemisphere) | 3858 | 9 | −59 | −8 | .0401 | |
| Crus II medial (left hemisphere) | 4309 | −6 | −82 | −29 | .0224 | |
| Lateral occipital (left hemisphere) | 10675 | −37 | −87 | −18 | 2.27×10−5 | |
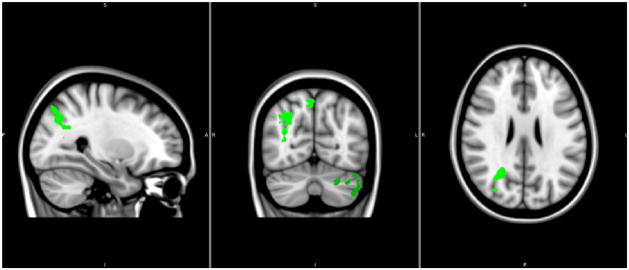
Figure 2.
Fluid reasoning > control condition: Significant group differences (control>ADHD) were limited to the right IPS and the left lateral cerebellum (Crus I and II) and are displayed in green (significant P cluster threshold <0.05; Z threshold >2.3). Higher Z thresholds (e.g., Z=4) while maintaining the same clusterwise significance level of p<0.05 (corrected for multiple comparisons) did not result in different spatial maps of significant group differences.
3. Discussion
Compared to controls, children with ADHD performed more poorly on a fluid reasoning task and demonstrated hypoactivation in regions critical for fluid reasoning including the intraparietal sulcus, which is implicated in visual attention and spatial processing, as well as the cerebellum, which is involved in executive functions such as cognitive planning, abstract reasoning, and attentional shifting through connections with the frontal lobe. Although there was some evidence of hypoactivation in frontal regions for the ADHD group compared to controls, these differences did not reach statisical significance. Further, individuals with ADHD performed as well as controls on the WISC-IV matrix reasoning subtest and the group differences in accuracy on the fluid reasoning condition of the fMRI task did not achieve statistical significance. Regardless, we did observe behavioral differences in response time with the ADHD group performing faster than controls as well as group differences in brain activation during fluid reasoning. Individuals with ADHD also performed the control condition less accurately than controls.
There is increasing evidence for the role of the intraparietal sulcus (IPS) role in spatial processing and allocating attentional resources (Landman et al., 2007; Vandenberghe et al., 2012). The IPS and frontal areas are part of a neural network thought to be responsible for top-down attentional control. Recent studies have identified structural connectivity between the posterior IPS and occipital cortex (Greenberg et al., 2012; Uddin et al., 2010) as well as a neuromodulatory role for IPS efferents on biasing visual processing and re-orienting of attention towards salient vs non-salient stimuli (Corbetta et al., 2008). This dorsal stream of attention regulation is likely to impact fluid reasoning as one needs to disengage from the previous solution set in order to focus on the next solution set. It has also been argued that the IPS is involved in the compilation of an attentional priority or saliency maps, i.e., a topographic representation of the distribution of attentional weights. In the presence of perceptually similar targets and distractors (like in the control condition), the calibration of attentional weights allows the observer to resolve the competition between simultaneously presented stimuli and will activate IPS (Vandenberghe et al., 2012); thus hypoactivation in this region for individuals with ADHD may be related to their significantly poorer performance in the control condition of the fluid reasoning task. Furthermore, the right IPS has been shown to be specifically involved in mental rotation tasks and visuospatial image transformations (Zacks, 2008), such as that which might be required to successfully perform the fluid reasoning condition of the task; thus hypoactivation in this region for individuals with ADHD may reflect more poorly developed mental rotation skills.
We also observed hypoactivation in the cerebellum in individuals with ADHD during fluid reasoning compared to controls. Specifically, the ADHD group showed less activation than controls in crus I and II extending to the inferior temporal gyrus and lateral occipital cortex. There is increasing evidence for the role of the cerebellum, particularly lobules VI and VII, in cognition and executive functioning as opposed to sensory or motor processing (Baldacara et al., 2008; O’Reilly et al., 2010; Stoodley and Schmahmann, 2009; Stoodley et al., 2010; Stoodley et al., 2011). This distinction has also been reported in studies of individuals with ADHD (Baldacara et al., 2008; Valera et al., 2005). Specifically, the literature suggests a role for the cerebellum in cognitive tasks that are difficult to solve, involve hypothesis generation or a self-determined solution, require planning and inferential processes to solve problems, or which occur in contexts of uncertainty (Kalbfleisch et al., 2007). The left Crus I has also been specifically implicated in mental rotation and complex decision making tasks (Stoodley et al., 2010). Hypoactivation in cerebellar regions for individuals with ADHD may also be related to the higher cogntive load in the experimental condition (Zang et al., 2005).
Taken together with evidence suggesting that cerebellar lobule VII and its hemispheric extensions crus I and crus II is reciprocally interconnected with the prefrontal and posterior parietal cortices (Stoodley et al., 2010), our findings suggest deficits in a prefrontal/occipito-parietal/cereballar loop during fluid reasoning in ADHD. Interestingly, this same network of regions is involved in timing disturbances in ADHD (Rubia et al., 2009); the faster reaction time on the fluid reasoning condition observed in the ADHD group compared to controls might reflect impulsive responding associated with hypoactivation in parieto-cerebellar regions. This is consistent with work suggesting widely distributed functional brain networks involving these regions are implicated in the pathophysiology of ADHD (Cao et al., 2006; Giedd et al., 2001; Wang et al., 2009). Future studies utlizing connectivity analyses are warranted to further investigate the relationship between these regions in fluid reasoning.
Somewhat surprisingly, we did not observe group differences in the rostrolateral prefrontal cortex despite observing differences, albeit non-signficant, in fluid reasoning accuracy. It may be that significant group differences would emerge with a larger sample size for both the behavioral and neuroimaging findings, paricularly in the rostrolateral prefrontal cortex which has been implicated as having a specific role in analogical reasoning, and inferior frontal gyrus which has a role in inhibition during fluid reasoning tasks (Watson and Chatterjee, 2012). Alternatively, deficits in relational reasoning in ADHD may be driven by deficits in ancillary cognitive processes, like allocation of attention versus deficits in relational reasoning abilities.
Although these results are suggestive, there are some limitations which make replication imperative. First, we utilized a block design which has inherent weaknesses including that it does not allow distinguishing between trial types within a block (e.g., correct versus error trials), does not account for the transient responses at the beginning and end of task blocks, and is known to be susceptible to habituation and changes in behavioral strategies between blocks (Petersen and Dubis, 2011). Also, the control condition for the fluid reasoning condition is not a tightly matched control but rather a low level control, which could result in activations potentially reflecting a diverse range of cognitive processes beyond perceptual matching (required of the control condition) and are required in the fluid reasoning task. These cognitive processes may include visuospatial attention, manipulation in working memory, relational integration, etc. While our sample is well characterized, it does include a mix of ADHD diagnostic subtypes which may manifest differential brain activation patterns (Solanto et al., 2009) which we were not able to directly investigate given our relatively small sample size. This study is the first, to our knowledge, to investigate fluid reasonining in ADHD utilizing fMRI. We show evidence of hypoactivation in regions known to be implicated in fluid reasoning in typically developing controls. Taken together with previous literature implicating these same regions in the pathogenesis of ADHD, our findings add to a burgeoning literature suggesting a neurological contribution to the executive functioning deficits observed in ADHD.
4. Experimental Procedure
4.1 Participants: Children with ADHD
Nineteen children with a primary diagnosis of ADHD between the ages of 8 and 12 (M=8.95 years, SD=1.18) were recruited from an ongoing randomized clinical trial investigating attention training to participate in a smaller fMRI pilot study (it should be noted that all participants were scanned prior to initiating attention training). Participants were all right-handed, not taking any psychotropic medications, and had an estimated full scale IQ score greater than 84 (i.e., less than 1 standard deviation below the mean). Children receiving alternative therapies to manage ADHD (e.g., biofeedback) in the same time frame as the intervention, with braces or any other metal contraindicated for fMRI, and/or with a history of serious head injury, current unstable medical or neurological conditions, suicidal or homicidal intent were excluded. Data from 7 children with ADHD were excluded (3 children were not able to complete the fMRI task because they fell asleep, 3 children demonstrated excessive movement during the fMRI scan, 1 child did not understand task directions). Table 1 lists the demographic characteristics for the final sample utilized in the fMRI analyses.
4.1 Participants: Typically Developing Controls
Ten children between the ages of 8 and 12 (M=9.4 years, SD=1.4) were recruited from local schools and the community. Participants were free from psychopathology based on the Behavioral Assessment of Children (BASC) (Reynolds and Kamphaus, 2004) rating scale (all T-scores < 65) and the ADHD items on the Swanson, Nolan, and Pelham ADHD Rating scale (SNAP-IV) (www.adhd.net) rating scale (Total score < 18; i.e., mostly ratings of just a little or not at all). Participants were all right handed and were not taking any psychotropic medications. Children with braces or any other metal contraindicated for fMRI were excluded. Participants also had an estimated full scale IQ score greater than 84.
4.3 Measures
Kiddie-SADS-Present and Lifetime Version (K-SADS-PL) (Kaufman et al., 1997)
Parents and children in the ADHD group were separately interviewed with the K-SADS-PL, a semi-structured interview of several DSM-IV diagnostic categories, to establish diagnosis of ADHD and rule out other comorbidities as part of the randomized clinical trial from which they were recruited (oppositional defiant disorder, separation anxiety disorder, specific phobia, and enuresis/encopresis were not exclusionary in the randomized clinical trial).
Wechsler Intelligence Scale for Children – Fourth Edition (WISC-IV) (Wechsler, 2004) Vocabulary and Block Design subtests
These subtests were administered to all participants to screen for at least average IQ (i.e., greater than 84) following procedures for pro-rating IQ from fewer subtests (Sattler and Dumont, 2004). The Matrix Reasoning subtest was also administered to assess fluid reasoning.
Behavioral Assessment Scale for Children (BASC) (Reynolds and Kamphaus, 2004)
Parents completed this standardized, normed measure on their children. Typically developing controls were required to have T-scores less than 65 for the Externalizing and Internalizing Composite scores and the Behavioral Symptoms Index.
Swanson, Nolan, and Pelham ADHD Rating scale (SNAP) (www.adhd.net)
Parents completed this 26 item measure describing their children on the 18 ADHD symptoms and 8 Oppositional Defiant Disorder symptoms on a scale of 0 (not at all) to 3 (very much). Typically developing controls were required to have a total score less than 18 on the ADHD items.
4.4 Procedure
Prior to participation, parents provided informed consent and children assented to participate in accordance with procedures established by the University of Texas Southwestern Medical Center Institutional Review Board. Imaging data were collected between June 2009 and December 2010 as part of a pilot study funded by the Children’s Medical Center Foundation at Dallas.
Children with ADHD were approached about study participation during the baseline visit of the ongoing randomized clinical trial, and interested families signed consent forms after the study was explained to them. ADHD diagnosis and other eligibility requirements were established as part of the randomized clinical trial. Typically developing controls who contacted study personnel in response to letters and flyers attended an appointment during which informed consent was obtained and eligibility established (i.e., parents completed behavioral ratings and children were administered IQ screening measures). Prior to the fMRI scan, all participants participated in a 30 to 60 minute mock scanning session to orient them to the scanning environment, the task, and the amount of tolerable movement. Immediately following the mock scan, participants completed their MRI and fMRI scans. Participants received $50 for their participation.
All scans were collected at the University of Texas Southwestern Medical Center. A Philips 3T fMRI scanner with a standard head coil was used with foam padding for head stabilization. Headphones were used to block scanner noise and to communicate with participants. All participants had access to a microphone in the scanner to communicate with study coordinator and technicians, and a panic device was placed next to their left hand to alert technicians in case of distress. After a brief survey, T1-weighted high-resolution 3-D anatomic images were obtained using a MPRAGE sequence with TR=8.1, TE=3.7, α=12, 160 sagittal 1mm slices to assist with localization. While participants completed the fluid reasoning task, gradient-echo echoplanar imaging (EPI) scan sequences were used to acquire BOLD function images; i.e., TR=2, TE=28, α=80, 40 axial 3mm slices, matrix=64×64, FOV=220×220×160, interleaved. Anatomical scans were obtained first, followed by the fluid reasoning paradigm.
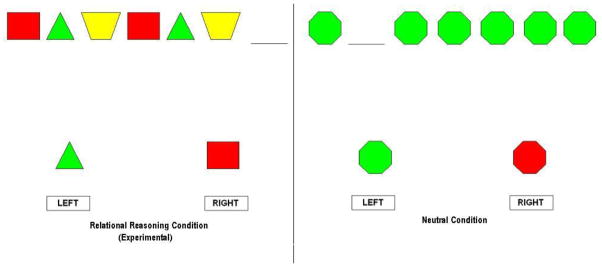
We utilized a fluid reasoning task previously used with typically developing children (Eslinger et al., 2008). The task involved 3 runs of a78 sec control, 78 sec experimental (fluid reasoning), and 15 sec rest conditions. The control and experimental blocks each consisted of 16 stimuli with a 4 sec exposure time and a .875 sec inter-stimulus interval blank screen. Children were asked to press a button designating which of two choices matched the stimuli along dimensions of color and shape. The experimental condition required fluid reasoning, while the control condition was a perceptual matching task controlling for activation to colors, shapes, motor response, etc., but did not involve a fluid reasoning demand. See Figure 3 for sample stimuli.
Figure 3.
Sample experimental and control stimuli.
Independent samples t-test (two-tailed) or chi square analyses were conducted comparing the two groups on demographic characteristics. Independent samples t-test comparisons (one-tailed) were conducted comparing the two groups’ behavioral performance (accuracy and reaction time) on the fluid reasoning task.
All fMRI data were analyzed using FEAT (FMRIB’s Expert Analysis Tool version 5.98), a module within the FSL processing package (FMRIB, Oxford, UK, www.fmrib.ox.ac.uk/fsl) used for preprocessing and model-based analysis of time series data. Within FEAT, standard pre-processing steps were handled first on the individual participant level: motion correction, slice timing correction, brain extraction, spatial smoothing, global intensity normalization, and high pass filtering. For low-level time series analyses, a General Linear Model method known as FILM (FMRIB’s Improved Linear Model) was used within FEAT to evaluate user-defined contrasts between explanatory variables defined in the design matrix (control > fluid reasoning; fluid reasoning >control; average ADHD response; average typically developing control response; age (demeaned); interaction between age and group). FILM uses a nonparametric estimation of the time series autocorrelation to pre-whiten each voxels’ time series, yielding improved estimation efficiency relative to methods which do not pre-whiten. Following the completion of FEAT, activation maps (e.g. parameter estimate maps converted to t-stat maps converted to z-stat maps with a threshold of 2.3 and a corrected cluster significance of p<0.05) associated with each contrast were available for display and review. For higher level (e.g. group-level) Bayesian mixed effects analyses, FMRIB’s FLAME (FMRIB’s local analysis of mixed effects) was used. FLAME uses Bayesian methods for modeling and estimating the random effects component (e.g. between-participants) and fixed effects component (e.g. within participants variability) of mixed effects variance using Metropolis-Hastings Markov Chain Monte Carlo sampling to estimate the true random effects variance and degrees of freedom at each voxel. A corrected cluster P threshold of <0.05 was used for each contrast. Significant clusters were then displayed on the study-specific template of high-resolution T1-weighted images for improved localization. A study-specific template of T1-weighted images facilitated transfer of significant clusters to the high-resolution MNI template used for coordinate information and display purposes in all figures. In contrast to purely fixed effects analyses, mixed effects modeling permits inferences to be made about the wider population from which the study participants were drawn.
Highlights.
Deficits in fluid reasoning may be related to cognition and behavior deficits in ADHD
This is the first fMRI study to investigate fluid reasoning in ADHD
Children with ADHD performed faster and less accurately than controls on the task
Results showed hypoactivation in ADHD in regions critical for fluid reasoning
Findings suggest particular deficits in parietal/cerebellar regions
Acknowledgments
The Children’s Medical Center Foundation at Dallas funded this pilot study through a Clinical Research Advisory Committee Award in partnership with the North Texas Clinical and Translational Science Initiative. The authors gratefully acknowledge the contributions of faculty and staff at UT Southwestern Medical Center including Lauren C. Smith, M.S., who assisted with recruitment and data collection, Conrad Barnes, B.A., who assisted with data management, F. Andrew Kozel, Ph.D. (now at the University of South Florida), who consulted regarding data analysis, and Hanzang Lu, Ph.D., who consulted with setting up the imaging paradigm. We are also grateful to the children who agreed to participate in the imaging study. Some of the data presented in this paper was previously presented as a poster presentation (Bush-Brassell et al., 2011).
Footnotes
Financial Disclosures
Drs. Tamm and Juranek report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Leanne Tamm, Email: leanne.tamm@cchmc.org.
Jenifer Juranek, Email: jenifer.juranek@uth.tmc.edu.
References
- American Psychological Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Author; Washington D.C: 1994. [Google Scholar]
- Baldacara L, Borgio JG, Lacerda AL, Jackowski AP. Cerebellum and psychiatric disorders. Revista brasileira de psiquiatria. 2008;30:281–9. doi: 10.1590/s1516-44462008000300016. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Berlin L, Bohlin G, Nyberg L, Janols LO. How well do measures of inhibition and other executive functions discriminate between children with ADHD and controls? Child Neuropsychol. 2004;10:1–13. doi: 10.1076/chin.10.1.1.26243. [DOI] [PubMed] [Google Scholar]
- Blair C. How similar are fluid cognition and general intelligence? A developmental neuroscience perspective on fluid cognition as an aspect of human cognitive ability. Behav Brain Sci. 2006;29:109–25. doi: 10.1017/S0140525X06009034. discussion 125–60. [DOI] [PubMed] [Google Scholar]
- Bush-Brassell A, Juranek J, Tamm L. Deficits in fluid reasoning associated with hypoactivation in ADHD: fMRI evidence. 22nd Eunethydis Meeting; Budapest, Hungary. 2011. [Google Scholar]
- Cao Q, Zang Y, Sun L, Sui M, Long X, Zou Q, Wang Y. Abnormal neural activity in children with attention deficit hyperactivity disorder: a resting-state functional magnetic resonance imaging study. Neuroreport. 2006;17:1033–6. doi: 10.1097/01.wnr.0000224769.92454.5d. [DOI] [PubMed] [Google Scholar]
- Carte ET, Nigg JT, Hinshaw SP. Neuropsychological functioning, motor speed, and language processing in boys with and without ADHD. J Abnorm Child Psychol. 1996;24:481–98. doi: 10.1007/BF01441570. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci. 2002;3:617–28. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Cho S, Moody TD, Fernandino L, Mumford JA, Poldrack RA, Cannon TD, Knowlton BJ, Holyoak KJ. Common and dissociable prefrontal loci associated with component mechanisms of analogical reasoning. Cerebral cortex. 2010;20:524–33. doi: 10.1093/cercor/bhp121. [DOI] [PubMed] [Google Scholar]
- Christoff K, Prabhakaran V, Dorfman J, Zhao Z, Kroger JK, Holyoak KJ, Gabrieli JD. Rostrolateral prefrontal cortex involvement in relational integration during reasoning. Neuroimage. 2001;14:1136–49. doi: 10.1006/nimg.2001.0922. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–24. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Rocha FF, Malloy-Diniz L, Lage NV, Romano-Silva MA, de Marco LA, Correa H. Decision-making impairment is related to serotonin transporter promoter polymorphism in a sample of patients with obsessive-compulsive disorder. Behavioural brain research. 2008;195:159–63. doi: 10.1016/j.bbr.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Dickstein SG, Bannon K, Xavier Castellanos F, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry. 2006;47:1051–62. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- Douglas V. Stop, look and listen: The problem of sustained attention and impulse control in hyperactive and normal children. Canadian Journal of Behavioral Science. 1972;4:259–281. [Google Scholar]
- Douglas V. Cognitive deficits in children with attention deficit disorder with hyperactivity. In: Bloomingdale LM, Sergeant J, editors. Attention Deficit Disorder: Criteria, Cognition, and Intervention. 1988. pp. 65–82. [Google Scholar]
- Eslinger PJ, Blair C, Wang J, Lipovsky B, Realmuto J, Baker D, Thorne S, Gamson D, Zimmerman E, Rohrer L, Yang QX. Developmental shifts in fMRI activations during visuospatial relational reasoning. Brain Cogn. 2008 doi: 10.1016/j.bandc.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Sanchez C, Estevez-Gonzalez A, Suarez-Romero E, Junque C. Right hemisphere dysfunction in subjects with attention-deficit disorder with and without hyperactivity. Journal of child neurology. 1997;12:107–15. doi: 10.1177/088307389701200207. [DOI] [PubMed] [Google Scholar]
- Geake JG, Hansen PC. Neural correlates of intelligence as revealed by fMRI of fluid analogies. Neuroimage. 2005;26:555–64. doi: 10.1016/j.neuroimage.2005.01.035. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Molloy E, Castellanos FX. Brain imaging of attention deficit/hyperactivity disorder. In: Wasserstein J, Wolf L, editors. Adult Attention Deficit Disorder: Brain Mechanisms and Life Outcomes. Annals of the New York Academy Sciences. Vol. 931. Academy of Sciences; New York: 2001. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan RJ. Functional neuroanatomy of three-term relational reasoning. Neuropsychologia. 2001;39:901–9. doi: 10.1016/s0028-3932(01)00024-0. [DOI] [PubMed] [Google Scholar]
- Gray JR, Chabris CF, Braver TS. Neural mechanisms of general fluid intelligence. Nat Neurosci. 2003;6:316–22. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- Greenberg AS, Verstynen T, Chiu YC, Yantis S, Schneider W, Behrmann M. Visuotopic Cortical Connectivity Underlying Attention Revealed with White-Matter Tractography. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:2773–2782. doi: 10.1523/JNEUROSCI.5419-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalbfleisch ML, Van Meter JW, Zeffiro TA. The influences of task difficulty and response correctness on neural systems supporting fluid reasoning. Cognitive neurodynamics. 2007;1:71–84. doi: 10.1007/s11571-006-9007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–987. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kerns KA, Eso K, Thompson J. Investigation of a direct intervention for improving attention in young children with ADHD. Developmental Neuropsychology. 1999;16:273–295. [Google Scholar]
- Klingberg T, Forssberg H, Westerberg H. Training of working memory in children with ADHD. Journal of Clinical and Experimental Neuropsychology. 2002;24:781–791. doi: 10.1076/jcen.24.6.781.8395. [DOI] [PubMed] [Google Scholar]
- Knauff M, Mulack T, Kassubek J, Salih HR, Greenlee MW. Spatial imagery in deductive reasoning: a functional MRI study. Brain Res Cogn Brain Res. 2002;13:203–12. doi: 10.1016/s0926-6410(01)00116-1. [DOI] [PubMed] [Google Scholar]
- Kroger JK, Sabb FW, Fales CL, Bookheimer SY, Cohen MS, Holyoak KJ. Recruitment of anterior dorsolateral prefrontal cortex in human reasoning: a parametric study of relational complexity. Cereb Cortex. 2002;12:477–85. doi: 10.1093/cercor/12.5.477. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Oosterlaan J, Stevenson J. Psychological mechanisms in hyperactivity: I. Response inhibition deficit, working memory impairment, delay aversion, or something else? J Child Psychol Psychiatry. 2001;42:199–210. [PubMed] [Google Scholar]
- Landman BA, Farrell JA, Jones CK, Smith SA, Prince JL, Mori S. Effects of diffusion weighting schemes on the reproducibility of DTI-derived fractional anisotropy, mean diffusivity, and principal eigenvector measurements at 1.5T. Neuroimage. 2007;36:1123–38. doi: 10.1016/j.neuroimage.2007.02.056. [DOI] [PubMed] [Google Scholar]
- Lee KH, Choi YY, Gray JR, Cho SH, Chae JH, Lee S, Kim K. Neural correlates of superior intelligence: stronger recruitment of posterior parietal cortex. Neuroimage. 2006;29:578–86. doi: 10.1016/j.neuroimage.2005.07.036. [DOI] [PubMed] [Google Scholar]
- Leth-Steenson C, Elbaz ZK, Douglas VI. Mean response times, variability, and skew in the responding of ADHD children: A response time distributional approach. Acta Psychologica. 2000;104:167–190. doi: 10.1016/s0001-6918(00)00019-6. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang Y. [Cognitive functions of children with attention deficit/hyperactivity disorder] Zhonghua Yi Xue Za Zhi. 2002;82:389–92. [PubMed] [Google Scholar]
- Lynn R, Meisenberg G, Mikk J, Williams A. National IQs predict differences in scholastic achievement in 67 countries. Journal of biosocial science. 2007;39:861–74. doi: 10.1017/S0021932007001964. [DOI] [PubMed] [Google Scholar]
- Ma L, Chen YH, Chen H, Liu YY, Wang YX. The function of hypothalamus-pituitary-adrenal axis in children with ADHD. Brain research. 2011;1368:159–62. doi: 10.1016/j.brainres.2010.10.045. [DOI] [PubMed] [Google Scholar]
- Marks DJ, Berwid OG, Santra A, Kera EC, Cyrulnik SE, Halperin JM. Neuropsychological correlates of ADHD symptoms in preschoolers. Neuropsychology. 2005;19:446–55. doi: 10.1037/0894-4105.19.4.446. [DOI] [PubMed] [Google Scholar]
- Morrison RG, Krawczyk DC, Holyoak KJ, Hummel JE, Chow TW, Miller BL, Knowlton BJ. A neurocomputational model of analogical reasoning and its breakdown in frontotemporal lobar degeneration. Journal of cognitive neuroscience. 2004;16:260–71. doi: 10.1162/089892904322984553. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Hinshaw SP, Halperin JM. Continuous performance test in boys with attention deficit hyperactivity disorder: Methylphenidate dose response and relations with observed behaviors. Journal of Clinical Child Psychology. 1996;25:330–340. [Google Scholar]
- Nigg JT, Quamma JP, Greenberg MT, Kusche CA. A two-year longitudinal study of neuropsychological and cognitive performance in relation to behavioral problems and competencies in elementary school children. J Abnorm Child Psychol. 1999;27:51–63. doi: 10.1023/a:1022614407893. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Is ADHD a disinhibitory disorder? Psychol Bull. 2001;127:571–98. doi: 10.1037/0033-2909.127.5.571. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Blaskey LG, Huang-Pollock CL, Rappley MD. Neuropsychological executive functions and DSM-IV ADHD subtypes. J Am Acad Child Adolesc Psychiatry. 2002;41:59–66. doi: 10.1097/00004583-200201000-00012. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Blaskey LG, Stawicki JA, Sachek J. Evaluating the endophenotype model of ADHD neuropsychological deficit: results for parents and siblings of children with ADHD combined and inattentive subtypes. J Abnorm Psychol. 2004;113:614–25. doi: 10.1037/0021-843X.113.4.614. [DOI] [PubMed] [Google Scholar]
- Nyden A, Billstedt E, Hjelmquist E, Gillberg C. Neurocognitive stability in Asperger syndrome, ADHD, and reading and writing disorder: a pilot study. Dev Med Child Neurol. 2001;43:165–71. [PubMed] [Google Scholar]
- O’Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen-Berg H. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cerebral cortex. 2010;20:953–65. doi: 10.1093/cercor/bhp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paloyelis Y, Mehta MA, Kuntsi J, Asherson P. Functional MRI in ADHD: a systematic literature review. Expert Rev Neurother. 2007;7:1337–56. doi: 10.1586/14737175.7.10.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasini A, Paloscia C, Alessandrelli R, Porfirio MC, Curatolo P. Attention and executive functions profile in drug naive ADHD subtypes. Brain Dev. 2007;29:400–8. doi: 10.1016/j.braindev.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. Journal of Child Psychology and Psychiatry. 1996;37:51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Dubis JW. The mixed block/event-related design. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2011.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakaran V, Smith JA, Desmond JE, Glover GH, Gabrieli JD. Neural substrates of fluid reasoning: an fMRI study of neocortical activation during performance of the Raven’s Progressive Matrices Test. Cognit Psychol. 1997;33:43–63. doi: 10.1006/cogp.1997.0659. [DOI] [PubMed] [Google Scholar]
- Raven JC. Advanced progressive matrices sets I and II. HK Lewis; London: 1965. [Google Scholar]
- Reynolds CR, Kamphaus RW. Behavior Assessment System for Children. 2. AGS Publishing; Circle Pines: 2004. Manual. [Google Scholar]
- Rubia K, Halari R, Christakou A, Taylor E. Impulsiveness as a timing disturbance: neurocognitive abnormalities in attention-deficit hyperactivity disorder during temporal processes and normalization with methylphenidate. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2009;364:1919–31. doi: 10.1098/rstb.2009.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Halari R, Cubillo A, Mohammad AM, Scott S, Brammer M. Disorder-specific inferior prefrontal hypofunction in boys with pure attention-deficit/hyperactivity disorder compared to boys with pure conduct disorder during cognitive flexibility. Human Brain Mapping. 2010;31:1823–33. doi: 10.1002/hbm.20975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda MR, Rothbart MK, McCandliss BD, Saccomanno L, Posner MI. Training, maturation, and genetic influences on the development of executive attention. Proc Natl Acad Sci U S A. 2005;102:14931–6. doi: 10.1073/pnas.0506897102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff CC, Knauff M, Fangmeier T, Spreer J. Reasoning and working memory: common and distinct neuronal processes. Neuropsychologia. 2003;41:1241–53. doi: 10.1016/s0028-3932(03)00016-2. [DOI] [PubMed] [Google Scholar]
- Sattler JM, Dumont R. Assessment of Children: WISC-IV and WPPSI-III Supplement. Jerome R. Sattler, Publisher, Inc; La Mesa: 2004. [Google Scholar]
- Schachar R, Logan G. Are hyperactive children deficient in attentional capacity? J Abnorm Child Psychol. 1990;18:493–513. doi: 10.1007/BF00911104. [DOI] [PubMed] [Google Scholar]
- Schafer V, Semrud-Clikeman M. Neuropsychological functioning in subgroups of children with and without social perception deficits and/or hyperactivity--impulsivity. J Atten Disord. 2008;12:177–90. doi: 10.1177/1087054707311662. [DOI] [PubMed] [Google Scholar]
- Semrud-Clikeman M, Walkowiak J, Wilkinson A, Christopher G. Neuropsychological differences among children with Asperger syndrome, nonverbal learning disabilities, attention deficit disorder, and controls. Developmental neuropsychology. 2010;35:582–600. doi: 10.1080/87565641.2010.494747. [DOI] [PubMed] [Google Scholar]
- Sergeant JA, Oosterlaan J, van der Meere J. Information processing and energetic factors in Attention-Deficit/Hyperactivity Disorder. In: Hogan HCQAE, editor. Handbook of Disruptive Behavior Disorders. Plenum Publishers; New York: 1999. pp. 75–104. [Google Scholar]
- Sergeant JA. The cognitive-energetic model: an empirical approach to attention-deficit hyperactivity disorder. Neurosci Biobehav Rev. 2000;24:7–12. doi: 10.1016/s0149-7634(99)00060-3. [DOI] [PubMed] [Google Scholar]
- Sergeant JA, Geurts H, Oosterlaan J. How specific is a deficit of executive functioning for attention-deficit/hyperactivity disorder? Behavioral Brain Research. 2002;130:3–28. doi: 10.1016/s0166-4328(01)00430-2. [DOI] [PubMed] [Google Scholar]
- Shallice T, Marzocchi GM, Coser S, Del Savio M, Meuter RF, Rumiati RI. Executive function profile of children with attention deficit hyperactivity disorder. Dev Neuropsychol. 2002;21:43–71. doi: 10.1207/S15326942DN2101_3. [DOI] [PubMed] [Google Scholar]
- Solanto MV, Schulz KP, Fan J, Tang CY, Newcorn JH. Event-related FMRI of inhibitory control in the predominantly inattentive and combined subtypes of ADHD. Journal of neuroimaging: official journal of the American Society of Neuroimaging. 2009;19:205–12. doi: 10.1111/j.1552-6569.2008.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Valera EM, Schmahmann JD. An fMRI study of intra-individual functional topography in the human cerebellum. Behavioural neurology. 2010;23:65–79. doi: 10.3233/BEN-2010-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: An fMRI study. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JM, Posner MI, Cantwell D, Wigal S, Crinella F, Filipek P, Emerson J, Tucker D, Nalgioclu O. Attention-deficit/Hyperactivity Disorder: Symptom domains, cognitive processes & neural networks. In: Parasuraman R, editor. The Attentive Brain. MIT Press; Boston: 1998. pp. 445–460. [Google Scholar]
- Tamm L, Menon V, Ringel J, Reiss AL. Event-related FMRI evidence of frontotemporal involvement in aberrant response inhibition and task switching in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2004;43:1430–40. doi: 10.1097/01.chi.0000140452.51205.8d. [DOI] [PubMed] [Google Scholar]
- Tamm L, Hughes C, Ames L, Pickering J, Silver CH, Stavinoha P, Castillo CL, Rintelmann J, Moore J, Foxwell A, Bolanos SG, Hines T, Nakonezny PA, Emslie G. Attention training for school-aged children with ADHD: results of an open trial. J Atten Disord. 2010;14:86–94. doi: 10.1177/1087054709347446. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Amin H, Rykhlevskaia E, Nguyen DA, Greicius MD, Menon V. Dissociable connectivity within human angular gyrus and intraparietal sulcus: evidence from functional and structural connectivity. Cerebral cortex. 2010;20:2636–46. doi: 10.1093/cercor/bhq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera EM, Faraone SV, Biederman J, Poldrack RA, Seidman LJ. Functional neuroanatomy of working memory in adults with attention-deficit/hyperactivity disorder. Biological psychiatry. 2005;57:439–47. doi: 10.1016/j.biopsych.2004.11.034. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Molenberghs P, Gillebert CR. Spatial attention deficits in humans: The critical role of superior compared to inferior parietal lesions. Neuropsychologia. 2012 doi: 10.1016/j.neuropsychologia.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Waltz JA, Knowlton BJ, Holyoak KJ, Boone K, Mishkin FS, Santos MM, Thomas CR, Miller BL. A system for relational reasoning in human prefrontal cortex. Psychological Science. 1999;10:119–125. [Google Scholar]
- Wang L, Zhu C, He Y, Zang Y, Cao Q, Zhang H, Zhong Q, Wang Y. Altered small-world brain functional networks in children with attention-deficit/hyperactivity disorder. Human Brain Mapping. 2009;30:638–49. doi: 10.1002/hbm.20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CE, Chatterjee A. A bilateral frontoparietal network underlies visuospatial analogical reasoning. Neuroimage. 2012;59:2831–8. doi: 10.1016/j.neuroimage.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Preschool and Primary Scale of Intelligence. 3. Harcourt Assessment, Inc; San Antonio: 2002. Administration Manual. [Google Scholar]
- Wechsler D. The Wechsler Intelligence Scale for Children. 4. Psychological Corporation; New York: 2003. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Intelligence Scale. 4. Harcourt Assessment, Inc; San Antonio: 2004. Administration Manual. [Google Scholar]
- Wendelken C, Nakhabenko D, Donohue SE, Carter CS, Bunge SA. “Brain is to thought as stomach is to ??”: investigating the role of rostrolateral prefrontal cortex in relational reasoning. J Cogn Neurosci. 2008;20:682–93. doi: 10.1162/jocn.2008.20055. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57:1336–46. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Zacks JM. Neuroimaging studies of mental rotation: a meta-analysis and review. Journal of cognitive neuroscience. 2008;20:1–19. doi: 10.1162/jocn.2008.20013. [DOI] [PubMed] [Google Scholar]
- Zang YF, Jin Z, Weng XC, Zhang L, Zeng YW, Yang L, Wang YF, Seidman LJ, Faraone SV. Functional MRI in attention-deficit hyperactivity disorder: evidence for hypofrontality. Brain & development. 2005;27:544–50. doi: 10.1016/j.braindev.2004.11.009. [DOI] [PubMed] [Google Scholar]