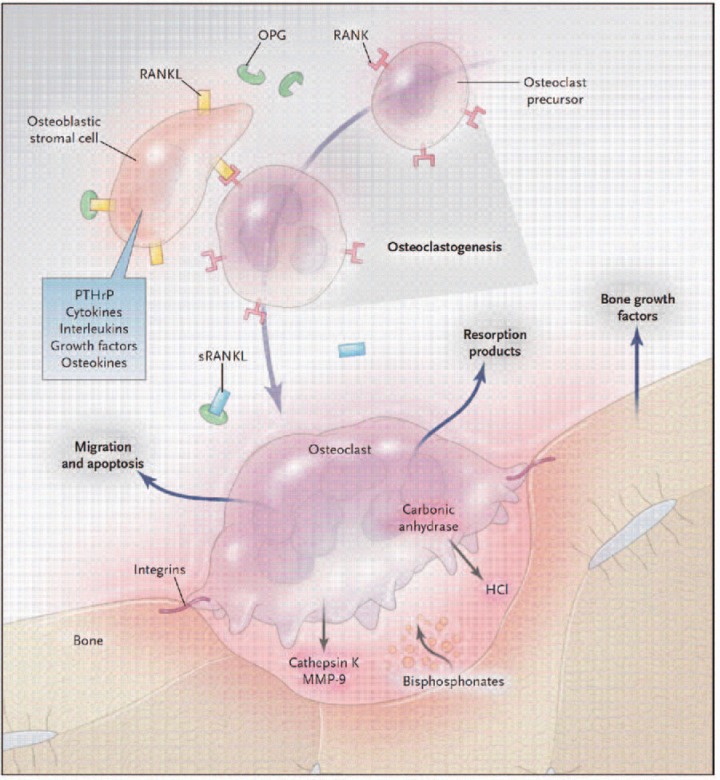
Figure 1.
Biology of the osteoclast in bone metabolism as a model for drug discovery. When bone is resorbed, growth factors in the bone matrix are released, often stimulating osteoclasts, which secrete resorption products into the circulation. Osteoblastic stromal (mesenchymal) cells and maturing osteoclasts express a soluble version of the osteokine-receptor activator of the nuclear factor KB ligand (sRANKL), its cell-bound receptor RANK, and osteoprotegerin (OPG), which sustain osteoclastogenesis. The stimulatory activity of RANKL and the inhibitory effects of OPG regulate osteolysis. Bisphosphonates concentrated under the osteoclasts inhibit resorptive function and promote osteoclast apoptosis. The incorporation of bisphosphonates into bone may increase resistance to resorption. Current therapies for hyperresorptive states can be accommodated by this model; the pathways outlined here suggest additional targets, for which therapies are under development: bone growth factor antagonists; guanine nucleotide exchange factors (GEFs), which inhibit cell migration; arginine—glycine—aspartic acid peptides, which act on integrins; carbonic anhy-drase inhibitors, which block the generation of hydrochloric acid; cathepsin K and matrix metalloproteinase 9 (MMP-9) inhibitors; and antibodies to parathyroid hormone-related protein (PTHrP) and RANKL. (With permission from Deftos LJ. (2005). Treatment of Paget's disease — taming the wild osteoclast. N Engt J Med 353: 872—875).