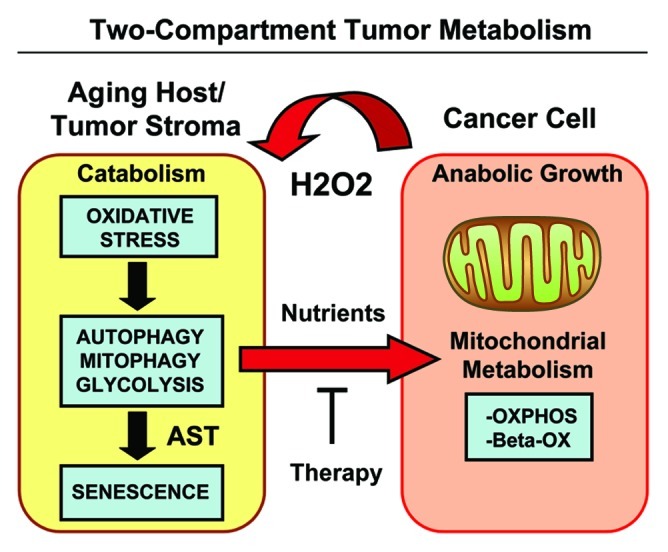
Figure 15. Two-compartment tumor metabolism is fueled by the autophagy-senescence transition (AST). In this model, cancer cells secrete hydrogen peroxide (H2O2), which induces oxidative stress in neighboring normal fibroblasts. Oxidative stress in stromal fibroblasts is then sufficient to confer the cancer-associated fibroblast phenotype, resulting in autophagy, mitophagy, and a shift toward aerobic glycolysis. Autophagy also drives the onset of senescence, via the autophagy-senescence transition. Autophagic-senescent fibroblasts then produce high-energy nutrients (L-lactate, ketone bodies, glutamine, and free fatty acids), which “fuel” mitochondrial metabolism (OXPHOS and β-OX; oxidative phosphorylation and β-oxidation) in adjacent cancer cells, resulting in the onset of anabolic tumor growth. This simple model could explain why chronological aging is one of the most significant risk factors for the development of cancer. AST, autophagy-senescence transition.
