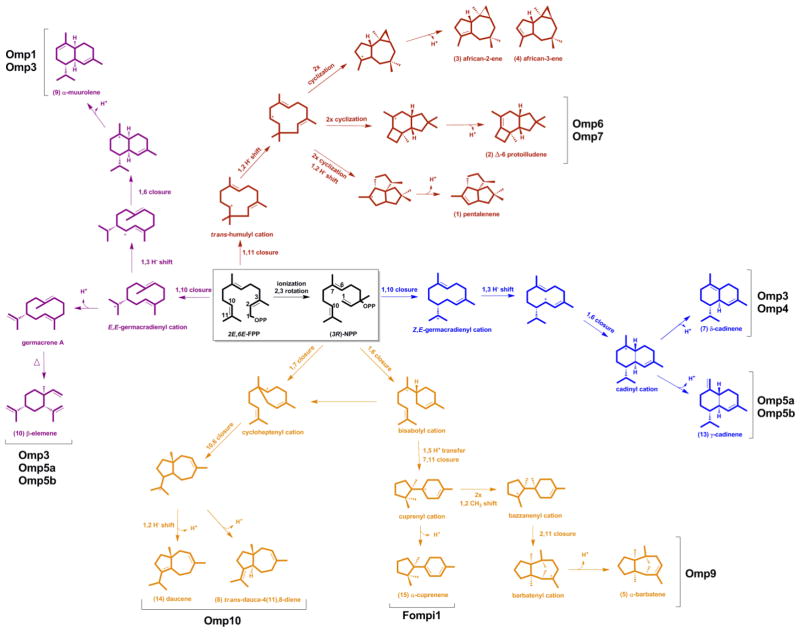
Figure 4.
Proposed cyclization pathways leading to O. olearius sesquiterpene products. Ionization of FPP results in a primary carbocation from (E,E)-FPP that can either undergo two different ring closures (1,10 or 1,11) or is isomerized to a secondary carbocation from (3R)-NPP, which can undergo four different ring closures (1,6; 1,7; 1,10 or 1,11). Shown are pathways leading to major sesquiterpene products identified in the culture headspace of O. olearius (pentalenene 1, Δ-6 protoilludene 2, african-2-ene 3, african-3-ene 4, α-barbatene 5, δ-cadinene 7, and trans-dauca-4(11),8-diene 8) and synthesized by recombinant Omp sesquiterpene synthases (Δ-6 protoilludene 2, α-barbatene 5, δ-cadinene 7, trans-dauca-4(11),8-diene 8, α-muurolene 9, β-elemene 10, γ-cadinene 13, and daucene 14) (see Figure S1 for compound mass spectra). Also shown is the cyclization pathway leading to α-cuprenene 15 synthesized by sesquiterpene synthase Fompi1 cloned from Fomitopsis pinicola.