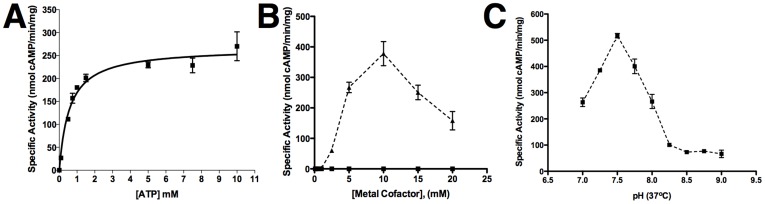
Figure 3. In vitro adenylyl cyclase activity of GST-PfACβ1-785.
(A) Adenylyl cyclase activity of purified GST-PfACβ1-785 was assessed with increasing concentrations of substrate ATP. Mn2+ was kept constant at 20 mM. The Michaelis constant was determined to be 0.57 mM (95% CI = 0.36 mM to 0.8 mM). Vmax was 266.7 nmol cAMP/min/mg (95% CI = 241.9 to 291.6). (B) Adenylyl cyclase activity was assessed over a range of Mn2+ (triangles; dotted line) and Mg2+ (squares; solid line) concentrations from 0.1 mM to 20 mM. ATP concentration was kept constant at 2.5 mM. Activity was only detectable with Mn2+ as a cofactor, and optimal Mn2+ was 10 mM providing a ratio of Mn2+:ATP = 4∶1. (C) pH optimum of GST-PfACβ1-785. Adenylyl cyclase assays were conducted over a pH range from 7 to 9 with 50 mM Tris buffer. A sharp pH optimum is evident at pH = 7.5. A shift in pH of 0.5 units resulted in a reduction of reaction velocity by ∼½.