Figure 1.
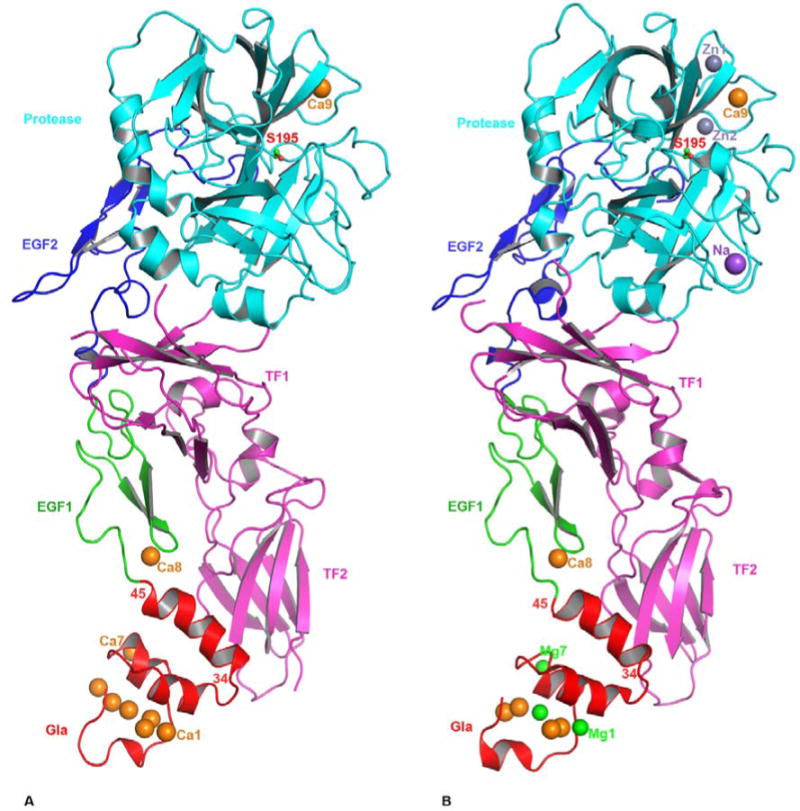
The cartoon representation of FVIIa/sTF complexes. The FVIIa/sTF structure of Banner et al., at 2.0 angstrom resolution (41) (PDB id 1DAN) in the presence of Ca2+ only is shown in A, and that of Bajaj et al., at 1.8 angstrom resolution (28) (PDB id 2A2Q) in the presence of Ca2+ and Mg2+ is shown in B. The locations of Na+ and Zn2+ sites are also shown in the 2A2Q structure. FVIIa consists of Gla (red), EGF1 (green), EGF2 (blue) and protease (cyan) domains, and the sTF (magenta) contains two fibronection type III domains. The different metal ions Ca2+(orange), Mg2+(green), Zn2+ (light blue) and Na+ (purple), bound to different domains of FVIIa are shown as spheres. Three Ca2+ ions in the GLA domain at positions 1, 4 and 7 (53) in the 1DAN structure are replaced by Mg2+ in the 2A2Q structure. The Ca2+ ion bound to the FVIIa EGF1 domain is labeled Ca8 and to the protease domain is labeled Ca9. In the 2A2Q structure Na+ ion is labeled as Na and the two Zn2+ ions labeled Zn1 and Zn2. The position of the active site Ser-195 is shown in ball and stick representation. The position of the C-terminal helix of the Gla domain (residues 34-45) of FVIIa is slightly tilted towards the TF2 domain in the 2A2Q structure resulting in a somewhat more favorable hydrophobic interactions between L13, F31 L39, and F40 of FVIIa with Y156, W158 and V207 of sTF. The residues interacting at the interface regions between FVIIa and sTF have been outlined in detail in an earlier report (41).
