Figure 6.
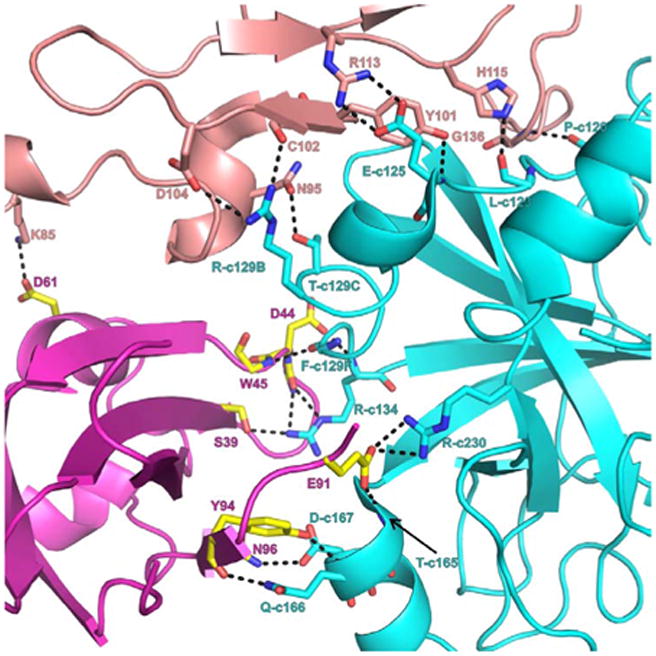
FVIIa-protease, FVIIa-EGF2 and sTF interface interactions. Interactive residues at the interface region of FVIIa protease domain (cyan), EGF2 domain (salmon) and sTF (magenta and yellow) are shown in stick representation. For clarity, either the side chain or main chain atoms that are involved in hydrogen bonding interactions are shown. The hydrogen bonds are represented by black dashed lines. Blue and red represent nitrogen and oxygen atoms, respectively. The residues N95, Y101, C102, D104, R113, H115 and G136 from EGF2 domain interact with P120, L123, E125, R129B and T129C of the protease domain. The residues S39, D44, W45, E91, Y94 and N96 of sTF interact with R134, T165, D167, Q166 and R230 of FVIIa-protease domain. The residue K85 of FVIIa-EGF2 domain makes hydrogen bond with D61 of sTF. Between the FVIIa-EGF2 and sTF, the hydrophobic interactions are more dominant (not shown). The chymotrypsin numbering scheme is used to label the residues.
