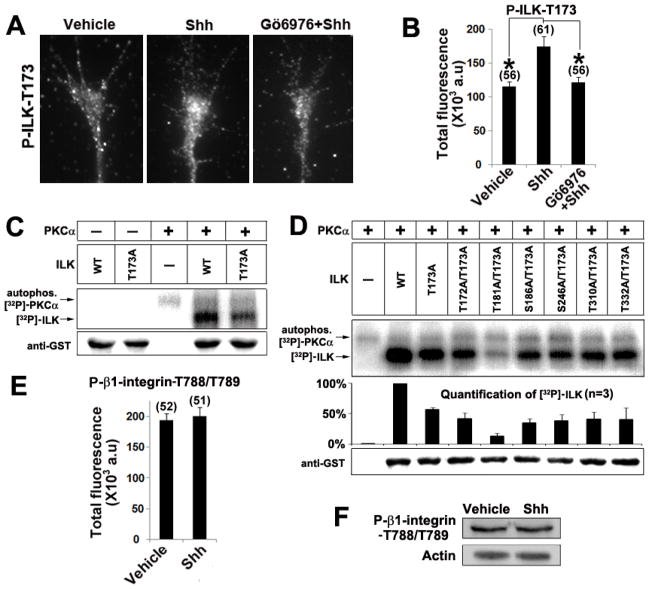
Figure 3.
Shh activates ILK through direct phosphorylation by PKCα. A, B, Chick RGC axon cultures were treated with vehicle control, Shh alone or Shh in the presence of Gö6976 for 2 mins. The immunofluorescent signals of phospho-ILK-T173 in each axon were quantified by ImageJ (see Methods) and the mean values were plotted. Numbers in parentheses indicate the total number of axons measured from three independent experiments. Data are represented as mean±SEM. *p<0.001, Student’s t test. C, In vitro kinase reactions were carried out by mixing the purified recombinant PKCα with wild type or ILK single mutant (ILK-T173A). Phosphorylation of ILK was analyzed on SDS-PAGE followed by autoradiography. The upper band shows the autophosphorylation of PKCα (81 kDa) and the lower band shows the phosphorylation of ILK-GST fusion protein (≈76kDa). Without PKCα, auto-phosphorylation of ILK-WT was undetectable. D, Double mutant (ILK-T173A/T181A) but not the other double mutations further reduced ILK phosphorylation to the background level. Equal loading of the gel was verified by western blot using an anti-GST antibody. E, Chick RGC axon cultures were treated with vehicle control or Shh for 2 mins. The immunofluorescent signal of phospho-β1-integrin (T788/T789) in each axon was quantified by ImageJ and the mean values were plotted. F, E6 chick retinas were treated with vehicle or Shh for 5 mins, cell lysates were run on SDS-PAGE gel and blotted with an antibody for phospho-β1-integrin (T788/T789).