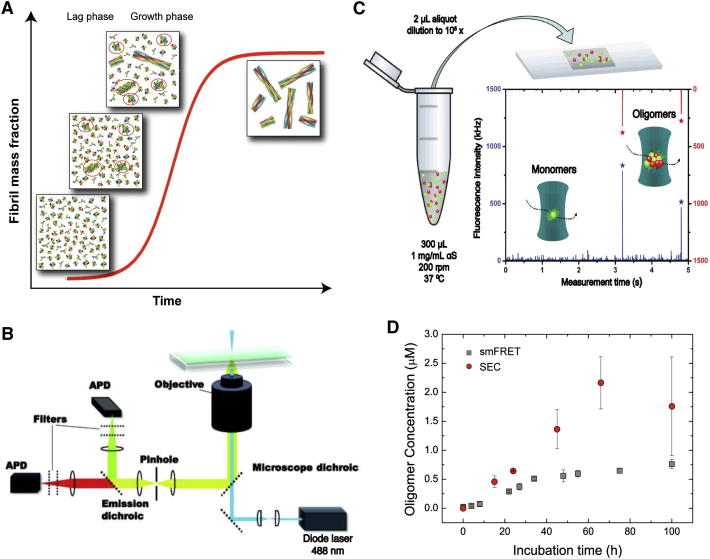
Figure 1.
Experimental Protocol
(A) Example of the kinetics of amyloid fibril formation, including hypothetical snapshots of the ensemble of αS species present at different phases of the aggregation process, wherein the oligomeric species present in the sample are highlighted with red circles.
(B) Schematic representation of the instrument used for smFRET measurements.
(C) Schematic description of the experimental protocol for aggregation experiments. Bursts of fluorescence coincident in both channels indicate the presence of FRET-positive oligomeric species (marked as asterisks). Noncoincident bursts can be attributed to monomers and are normally much less bright than those corresponding to oligomers.
(D) Comparison between the kinetics of oligomer formation under bulk conditions obtained by quantitative SEC analysis (red circles; see also Figure S1G) and from smFRET experiments, after extrapolating the concentrations from sm to bulk conditions (gray squares). The data reported correspond to the mean and standard errors of two repetitions in the case of the SEC data and five repetitions for the sm data. See Figure S1 for a detailed characterization of the effect of the fluorophores on αS aggregation.