Figure 5.
Kinetic Analysis of αS Oligomerization
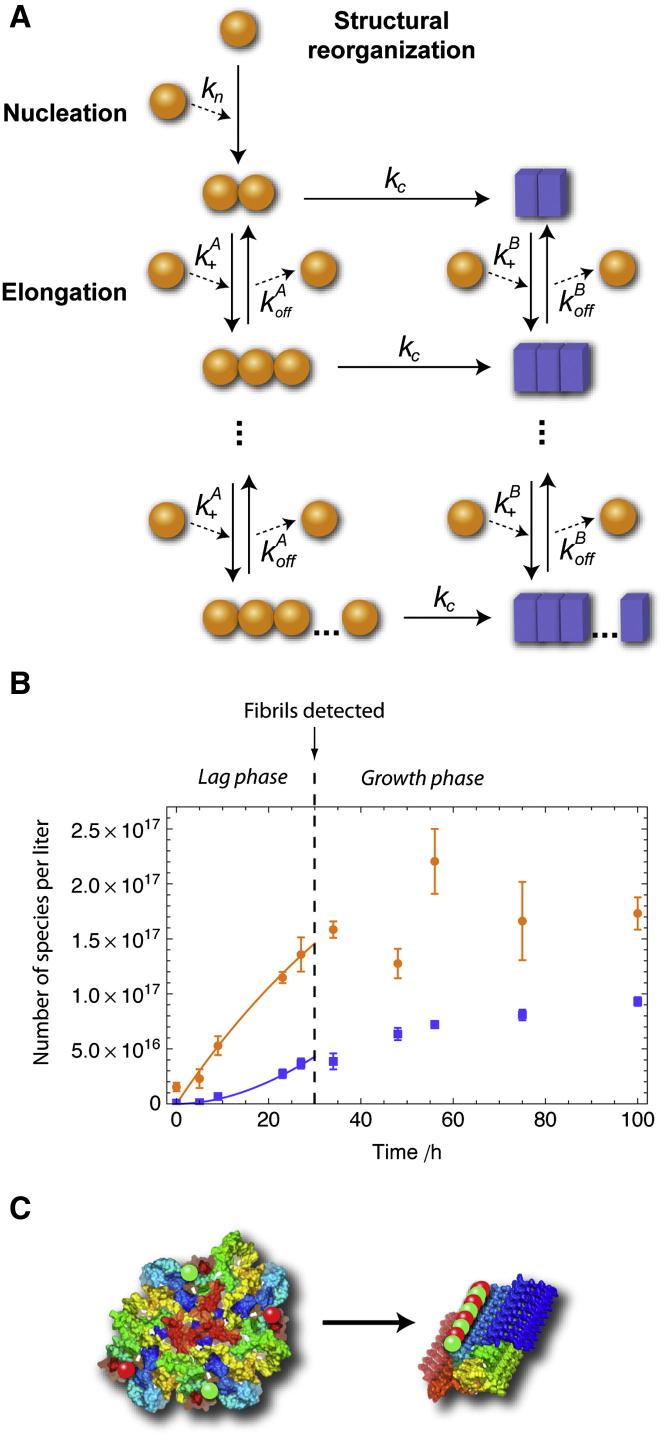
(A) Scheme for the minimalistic kinetic model used to fit the early stages of αS aggregation.
(B) Results of the global fitting (continuous lines) of the kinetics of formation of the two types of oligomeric species estimated under bulk conditions from smFRET experiments. Data for type A oligomers and type B oligomers are shown as orange circles and blue squares, respectively (average and standard error of five different experiments). The vertical dashed line is at 30 hr, corresponding to the lag phase of fibril formation estimated under bulk conditions, up to which time our model accounts well for the different microscopic processes governing the aggregation reaction.
(C) Cartoon showing the conversion of an 8-mer of αS from a collapsed to an ordered proteinase-K-resistant form. Residues of each monomer are colored according to their location in the amino acid sequence. The average distance between fluorophores, represented as green and red spheres, is different for each type of oligomer and hence gives rise to different average FRET efficiencies.