Abstract
Ancestral environmental exposures have previously been shown to promote epigenetic transgenerational inheritance and influence all aspects of an individual’s life history. In addition, proximate life events such as chronic stress have documented effects on the development of physiological, neural, and behavioral phenotypes in adulthood. We used a systems biology approach to investigate in male rats the interaction of the ancestral modifications carried transgenerationally in the germ line and the proximate modifications involving chronic restraint stress during adolescence. We find that a single exposure to a common-use fungicide (vinclozolin) three generations removed alters the physiology, behavior, metabolic activity, and transcriptome in discrete brain nuclei in descendant males, causing them to respond differently to chronic restraint stress. This alteration of baseline brain development promotes a change in neural genomic activity that correlates with changes in physiology and behavior, revealing the interaction of genetics, environment, and epigenetic transgenerational inheritance in the shaping of the adult phenotype. This is an important demonstration in an animal that ancestral exposure to an environmental compound modifies how descendants of these progenitor individuals perceive and respond to a stress challenge experienced during their own life history.
Phenotype is determined by both inherited and experienced factors. Traditionally, the former are regarded as a result of genetic inheritance, and the latter encompass events in the individual’s personal life history. Study of how the environment shapes the phenotype was initially referred to as “epigenesis” (1) and later termed “epigenetics” by Waddington (2). The current definition for epigenetics used in this study is “molecular factors or processes that regulate genome activity independent of DNA sequence and are mitotically stable” (3). The model used in the current study involves an epigenetic transgenerational inheritance of a behavioral phenotype (4) induced by an environmental toxicant (5) and transmitted through the germ line, involving a permanent alteration in the sperm epigenome (i.e., DNA methylation) (6). The epigenetic transgenerational inheritance of this altered sperm epigenome modifies the subsequent development and epigenomes of all cells and tissues, including the brain, to promote phenotypic variation (7). Although no direct epigenetic measurements were made in the current study, the epigenetic model and role of epigenetics in development provides the molecular basis of the observations presented.
The development of brain and behavior involves at least two distinct epigenetic programming mechanisms (3, 8). “Germ line-dependent” epigenetic change occurs when the modified epigenome is permanently incorporated into the germ line to manifest each generation in the absence of the causative agent. “Context-dependent” epigenetic change occurs when the environmental factors that bring about the epigenetic modification persist in the environment. Most research in epigenetics today falls within this context-dependent category. Although both have been attributed with “generational” properties, only germ line-dependent epigenetic modification is epigenetic transgenerational inheritance (5, 7). The life-history approach to the study of behavioral development emphasizes both the continuity and interplay between the internal and external environmental characteristic of the specific life stages. Most research on the effects of stress has focused on the earliest life stages (fetus and neonate) or adulthood, with relatively few studies on adolescence (9–11). It is during this period that adrenarche and pubarche occur and the individual graduates from dependence to independence, assuming the properties of maturity. Stress experienced during adolescence has enduring effects, including neural remodeling, sensitivity to drugs of abuse, impaired learning and memory, and altered emotional behaviors in adulthood (12–15). The current study shows that the effects of chronic restraint stress (CRS) during adolescence on the adult physiological, behavioral, and neural phenotypes become more profound when considered in the context of epigenetic transgenerational inheritance.
We investigated this complex phenotypic response with a unique statistical approach for multidimensional phenotype analysis (16). Systems biology attempts to understand how molecular- to organism-level processes are involved in the emergence of complex phenotypes. Emergence was originally formulated by Weiss (17, 18) to mean “phenotypes, and the mechanisms that underlie them, depend on, and subordinate to, the law which rules the complex as a unit.” Systems biology approaches have recently been used to examine the phenotype at the molecular level of genetics or epigenetics (19). The current study tests the hypothesis that a combination of an environmentally induced epigenetic transgenerational inheritance (lineage) and context-dependent stress (stress) interact and promote alterations in brain development and genome activity (gene networks) that alter the adult phenotype at all levels.
Results
In social animals, the presence of conspecifics is another powerful force shaping how an individual responds to abiotic and biotic stimuli (Fig. S1A). When housed together individuals tend to be less sensitive to stressors that, if experienced alone, are debilitating or lethal (20). Social status also influences sensitivity to negative allostatic factors, with subordinate individuals often faring more poorly than dominant individuals. Social housing also modulates both the stress response (21) and the behavior of transgenerationally epigenetically modified individuals (22). Thus, individuals from each lineage were housed together in dyads; half of the dyads were exposed to CRS.
Physiological Phenotype.
Body weight (BW) and gonadosomatic and adrenosomatic indices.
The pattern of BW gain differs according to lineage and stress (Fig. S1B). There is no difference in BW between vinclozolin-lineage (V-L) and control-lineage (C-L) males at birth or at weaning. As expected, shipping stress depresses BW in all animals. The effect of lineage is observed in the nonstress groups with V-L males gaining weight more rapidly and becoming heavier than C-L males (P = 0.02). CRS depresses gain in both lineages: within 2 d of onset of CRS, weight gain (average percentage gain relative to previous weight) in stress animals is half that of nonstress males regardless of lineage; on the cessation of CRS, BW increases in both lineages. Interestingly, there is no interaction of lineage and stress. Finally, stress attenuates the difference in BW between the lineages: in the nonstress dyads, V-L males are ∼25 g heavier than C-L males are, whereas, in the stress dyads, the weight differences are less than 5 g (Fig. S1B Inset). There are no lineage effects in gonadosomatic or adrenosomatic indices, but males experiencing CRS have larger testes than do nonstress males in the V-L group and V-L stress males have larger adrenals than C-L stress males do (both P = 0.03). When considering the dyad, males in stress dyads have larger testes and adrenals (P = 0.03 and 0.02, respectively) than do males in nonstress dyads. Finally, within-dyad analysis reveals that, in the stress dyads, V-L males have larger adrenals than do C-L males (P = 0.01); this effect is not seen in nonstress dyads (Fig. S2A).
Hormonal indices.
With a single exception, CRS results in lower corticosterone (CORT) levels in both the C-L and V-L groups (Fig. S1C). Lineage, but not stress, influences circulating testosterone (TESTO) levels (F1, 63 = 2.11, P = 0.04). In stress animals, TESTO levels are significantly higher in V-L males relative to C-L males (P = 0.01). There is no significant difference in circulating concentrations in leptin across lineage or stress conditions, perhaps because BW is stable and unchanging by the time of death [postnatal day (PND) 120].
Behavioral Phenotype.
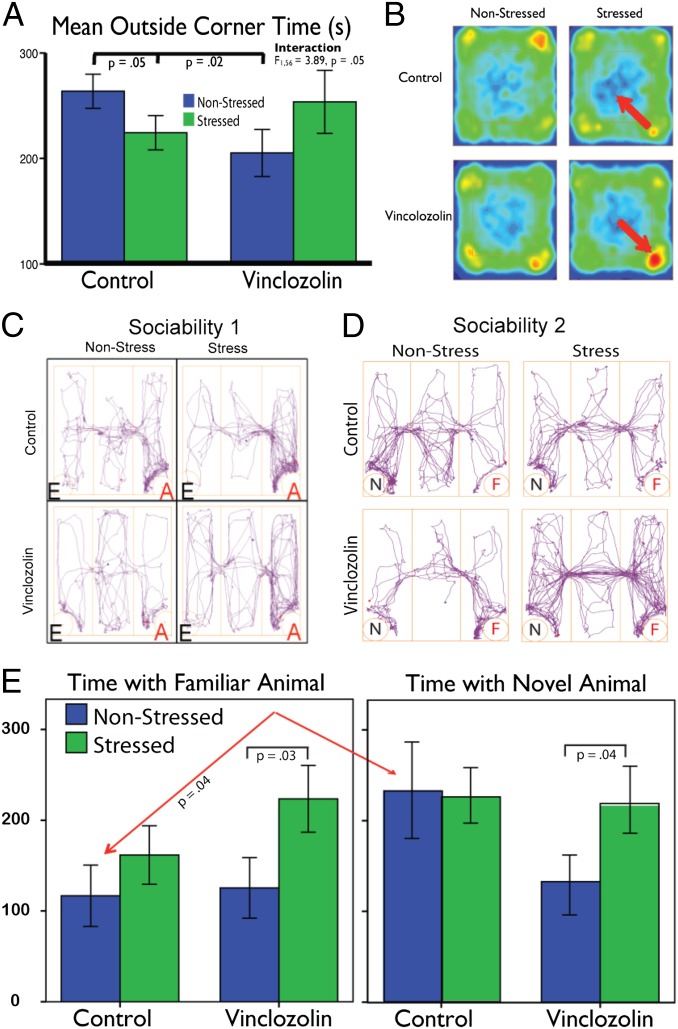
In the forced-swim (FS) test, there is no difference according to lineage or stress condition in terms of distance, speed, latency to immobility, or time mobile measures, even after controlling for BW differences. In the open-field (OF) test, C-L nonstress males spend more time in the corners of the OF than do V-L nonstress males (Fig. 1A). Exposure to CRS has opposite effects in the two lineages (Fig. 1A): C-L males move out of corners and into the center, indicating greater exploration, whereas V-L males move from the center into corners, indicating greater anxiety [interaction between lineage and stress (F1, 56 = 3.89, P = 0.05)] (Fig. 1B). Considering the dyad, V-L males in nonstress condition cross the center more frequently (P = 0.01), whereas, in the stress condition, they cross the center faster (P = 0.04) than C-L males do. There is also an effect of stress independent of lineage: stressed males move faster through the center than do nonstressed males (P = 0.01), indicating that CRS increases anxiety later in adulthood (Fig. S2B).
Fig. 1.
Behavior analysis. (A) In OF tests, C-L nonstress males spent more time in corners than did V-L nonstress males. (B) Heat map showing occupancy for group means in the OF from the bird’s eye view. Red indicates greater time spent at any given position. Arrows indicate change in activity as a consequence of stress. (C) Overhead view of group mean tracing of movement within a schematic of the testing chamber for animals in Soc 1. “E” indicates an empty stimulus cage; “A” indicates a stimulus cage containing an animal. (D) Overhead view of group mean tracing of movement within a schematic of the testing chamber for animals in Soc 2. “N” indicates a stimulus cage containing a novel male; “F” indicates a stimulus cage containing a familiar animal. (E) Evidence of transgenerational epigenetic modification on response to CRS on social bonding.
In sociability test 1 (Soc 1) (Fig. 1C), lineage effects are restricted to the stress dyads, with V-L males traveling farther and faster than the C-L males do (both P = 0.04) and choosing to associate with the stimulus animal more than nonstress individuals (P = 0.03). In general, CRS affects line crossing (P = 0.04) and latency to first entry into the chamber containing the stimulus animal (P = 0.01). In the nonstress dyads, V-L males visit the stimulus animal for longer periods and move between chambers less than did C-L males do (both P = 0.04) (Fig. S2B).
In Soc 2, C-L nonstress males spend more time with the novel stimulus male than with the familiar stimulus male (P = 0.01) (Fig. 1 D and E). Only V-L males show effects of stress, traveling farther (P = 0.04) and faster (P = 0.05) than V-L nonstress males do; they also spend less time in the center compartment (P = 0.01) and more time with the familiar and novel stimulus males (P = 0.03). Comparison of the two tests reveals that, in Soc 1, V-L stress males tend to spend less time in the center compartment than do V-L nonstress males, a difference that becomes significant in Soc 2 (P = 0.01), suggesting that V-L stress males display greater affiliation behavior with the familiar individual. In C-L males, there is no effect of stress, but mean center time decreases in Soc 2, a difference significant only in the stress condition (P = 0.03). Similarly, V-L stress males tend to spend more time in the animal chamber in Soc 1 than do V-L nonstress males and in Soc 2; this difference becomes significant (P = 0.03), again suggesting formation of a social bond with the familiar animal. See Fig. 2B and Fig. S2B for landscape analysis.
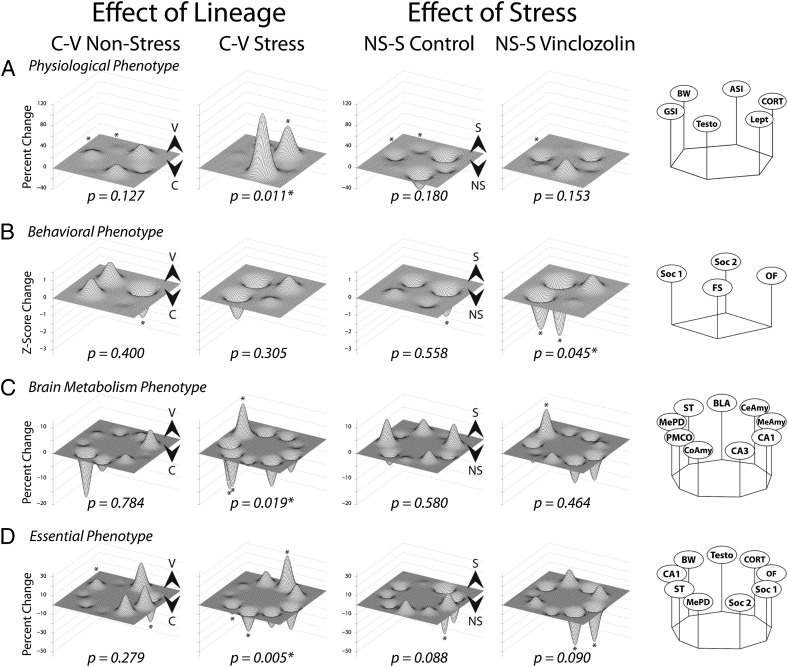
Fig. 2.
Phenotype analysis at different levels of biological organization. Leftmost columns depict effects of lineage (difference between C-L and V-L) under nonstress and stress conditions. An asterisk above a peak or a valley indicates a significant effect of treatment in that behavioral test (P < 0.05). Differences in phenotype calculated by permutation analysis on this dataset yielded the p results shown beneath each landscape, indicating the degree to which the landscape is changed. A peak for a trait indicates a greater result in V-L (V ) males, whereas a valley indicates a greater result in C-L (C
) males, whereas a valley indicates a greater result in C-L (C ) males. Rightmost columns depict effects of stress (difference between nonstress and stress) in C-L and V-L males. A peak for a trait indicates a greater result in stress (S
) males. Rightmost columns depict effects of stress (difference between nonstress and stress) in C-L and V-L males. A peak for a trait indicates a greater result in stress (S ) conditions, whereas a valley indicates a greater result in nonstress (NS
) conditions, whereas a valley indicates a greater result in nonstress (NS ) conditions. Nodes represent group means of percentage maximum or Z scores (see SI Materials and Methods for specifics). (A) Body phenotype. Clockwise nodes: BW; ASI, adrenosomatic index; CORT; Lept, leptin level; TESTO; and GSI, gonadosomatic index. (B) Behavior phenotype. Clockwise nodes: Soc 2, measure of social novelty and working memory; OF; FS; and Soc 1, measure of social approach, anxiety, and exploration. (C) Brain metabolism phenotype. Clockwise nodes: BLA, CeAmy, MeAmy, CA1, CA3, CoAmy, PMCo, MePD, and ST. (D) Essential phenotype or the three most influential measures from each category (physiology, behavior, and brain). Clockwise nodes: TESTO, CORT, OF, Soc 1, Soc 2, MePD metabolic activity, ST metabolic activity, CA1 metabolic activity, and BW.
) conditions. Nodes represent group means of percentage maximum or Z scores (see SI Materials and Methods for specifics). (A) Body phenotype. Clockwise nodes: BW; ASI, adrenosomatic index; CORT; Lept, leptin level; TESTO; and GSI, gonadosomatic index. (B) Behavior phenotype. Clockwise nodes: Soc 2, measure of social novelty and working memory; OF; FS; and Soc 1, measure of social approach, anxiety, and exploration. (C) Brain metabolism phenotype. Clockwise nodes: BLA, CeAmy, MeAmy, CA1, CA3, CoAmy, PMCo, MePD, and ST. (D) Essential phenotype or the three most influential measures from each category (physiology, behavior, and brain). Clockwise nodes: TESTO, CORT, OF, Soc 1, Soc 2, MePD metabolic activity, ST metabolic activity, CA1 metabolic activity, and BW.
Brain Metabolism Phenotype.
Previous research on the neural and behavioral consequences of CRS has identified 15 brain regions that play a role in stress reactivity as well as learning and memory (Tables S1 and S2 ). Assessing metabolic activity by using cytochrome histochemistry (23) in these nuclei, we find that nine nuclei capture 87% of the variance: basolateral amygdala (BLA), medial amygdala (MeAmy), central amygdala (CeAmy), anterior cortical amygdala (CoAmy), posteromedial cortical amygdala (PMCo), medial posterior dorsal amygdala (MePD), stria terminalis (ST), and CA1 and CA3 areas of the hippocampus (Table S2). V-L males subjected to CRS show an approximately 10% decrease in overall activity in the brain, but other experimental groups are relatively unchanged (Fig. 2C and Fig. S2C and Tables S1 and S2). Specific nuclei show both lineage and stress effects. The PMCo V-L males in both stress and nonstress groups show a substantial decrease (13% and 17%, respectively) in metabolic activity within the PMCo (P = 0.03,0.04, respectively) relative to C-L males. In the MeAmy, C-L stress males show an 8.4% increase in activity. In the MePD, V-L stress males show a marked decrease in activity relative to C-L stress males (∼18%, P < 0.01), whereas, in C-L stress males, there was only a 9% increase in activity. In the ST, V-L males that have been subjected to CRS show an ∼12% increase in activity relative to C-L males also subjected to CRS (P = 0.02). In V-L males, CRS results in a 10% increase in activity (P = 0.05). The CA1 and CA3 of the hippocampus show effects similar to one another. In animals that have been subjected to CRS, the CA1 shows an ∼7% decrease in activity, and the CA3 shows an 11% decrease in activity in V-L animals relative to C-L males. Similarly, in V-L animals, males that were subjected to CRS show a 10% decrease in activity in the CA1 and an ∼9% decrease in activity in the CA3 relative to males that were not subjected to CRS. This effect indicates a general decrease in activity within the hippocampus in animals that have been subjected to both vinclozolin and CRS, whereas animals that were subjected to either vinclozolin or CRS (but not both) do not show great changes in activity. See Fig. S2C for landscape analysis.
Essential Phenotype.
An essential phenotype landscape was constructed by combining the three measures from each level of analysis that best differentiate between groups as determined by discriminant function analysis (DFA) followed by permutation analysis. This analysis determined how changes within and between phenotype classes are correlated with and separate from the effects of lineage versus the effects of CRS (Fig. 2D), revealing (i) no significant difference between C-L and V-L males; (ii) V-L males perceive and respond to CRS differently than do C-L males; and (iii) CRS affects males of both lineages to the same approximate degree, albeit it in different ways on different traits.
Brain Genomics and Gene Networks.
For the genomic and gene network studies, RNA was derived from 1-mm punches from the CA1 and CA3 of the hippocampus, BLA, and the primary and secondary motor cortex (CRTX) from each individual (Fig. S3). The comparisons made to assess alterations in gene expression are shown in Table S3, with the C-L nonstress males considered as the baseline or “normal” brain. The number of genes with significant differences in expression with a minimum fold change of 1.2 (fold change > 1.2) and mean difference of >10 are presented for all comparisons in Table S3 and Dataset S1. The altered gene sets are each given a list number 1–16 and involve 23–377 genes, depending on the comparison and brain region analyzed. Each brain region, both within and between lineage and stress conditions, has its own set of distinct genes with negligible overlap. Indeed, only a single gene, ribosomal protein L21, is common (Fig. S4). A complete list of the genes with altered expression for each brain region and comparison is presented in Dataset S1 (lists 1–16) and categorized to various cellular functions and processes (Fig. S5), with receptors and binding proteins, metabolism, transcription, signal transduction, and development being highly represented. Various comparisons of lineage and stress conditions have genes that share common gene functional categories but are independent in their patterns of change according to lineage and stress. Analysis of the altered gene sets correlated to specific signaling pathways, and cellular processes show the top 20 pathways in multiple comparative lists (Tables S3 and S4). The highest correlated pathway is olfactory transduction, with 78 genes altered among all of the comparisons (Fig. S6). Nearly all of the comparative gene lists had the highest number of altered genes in this pathway. Other brain-related pathways affected by lineage and stress are neuroactive ligand–receptor interaction, Huntington disease, Alzheimer’s disease, axon guidance, and Parkinson disease (Tables S3 and S4). Two of the more ubiquitous pathways affected are the calcium signaling pathway and the MAPK signaling pathway (Fig. S7). Although unique gene sets exist for each brain region analyzed, the lineage and stress altered gene sets are associated with common signaling pathways and cellular processes. Interestingly, a number of brain-specific pathways associated with neurodegenerative disease were identified.
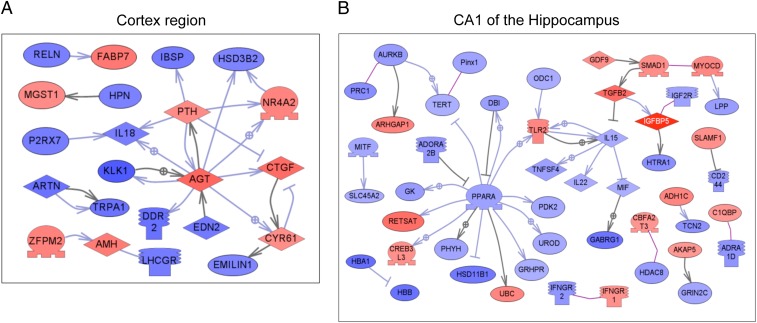
The final genomic analysis of the brain region transcriptome comparisons involved identification of gene networks by using global literature analysis software with the altered brain gene sets (24). The direct-connection (i.e., interaction) gene networks were identified for the CRTX and the CA1 regions (Fig. 3). These networks identify how the genes with altered expression are connected and associated with the changes in the brain regions and behavior. Interestingly, the two regions have distinct networks. The BLA and the CA3 have direct neural and gene network connections, and their individually identified gene networks also identify the indirectly connected genes and cellular processes they regulate. The direct-connection gene networks of the CRTX and CA1 provide novel networks of genes involved in the regulation of the brain regions and behavioral alterations. The gene networks identified involve a combination of lineage and stress factors that cannot be segregated. Similar analysis for each of the individual comparisons of lineage and stress for each region separately demonstrated no other major direct-connection gene network, but all had indirectly connected gene networks. Thus, each of the gene networks identified is unique and specific to brain region, lineage, or stress condition. Observations indicate that each brain region has a different altered gene set and gene network involved in the lineage and stress comparisons. These gene networks are directly associated with the behavioral alterations observed and are speculated to, in part, be causally related. Because neither the lineage nor the stress effects promote genetic mutations, but they do promote epigenetic alterations (7), epigenetics is the basal molecular process involved, as previously documented (6). Therefore, the germ line-dependent transgenerational modification and the context-dependent stress response modification promotes an epigenetic reprogramming of these brain regions that alters the gene networks and pathways identified to promote the altered behavioral phenotypes observed.
Fig. 3.
Direct-connection networks for genes in CRTX (gene lists 5–8; A) or CA1 (gene lists 9–12; B) obtained by global literature analysis using Pathway Studio 7.0 software (Ariadne Genomics). (A) For cortex, only 22 directly connected genes of 330 unique genes (no ESTs included) from combined lists 1–4 are shown. (B) For CA1, 47 genes of 430 unique genes (no ESTs included) from combined lists 9–12 are shown. The rest of genes are not connected and not shown.
A validation of the gene expression of the microarray data was performed with a quantitative PCR (qPCR) analysis of selected genes from the gene networks that were highly connected and critical to the regulation of the gene network. These genes were angiotensinogen (Agt) and connective tissue growth factor (Ctgf) (Fig. 3A), insulin-like growth factor binding protein 5 (Igfbp5) (Fig. 3B), and brain-derived neurotrophic factor (Bdnf) (Fig. S8A). The altered gene expression for all four genes had similar trends for the microarray and qPCR (Agt: 1.34 vs. 1.6; Ctgf: 1.31 vs. 1.92; Igfbp5: 1.70 vs. 1.36; and Bdnf: 1.21 vs. 1.63, respectively), and all had statistically significant differences (P < 0.05). Therefore, the qPCR validated the microarray data for these critical genes in the various gene networks identified.
Discussion
Our observations illustrate a “two-hit” model where the “hits” span generations, in this instance the first hit (transgenerational epigenetic inheritance) predisposing a future generation to respond to a second hit (CRS during second adolescence), which further alters the adult phenotype. The first hit of this model corresponds to the exposure of a gestating female to the fungicide vinclozolin occurring three generations earlier. As demonstrated previously, vinclozolin exposure predisposes males to develop a variety of adult-onset diseases earlier than normal (5, 7), effects still detectable in males over four subsequent generations without diminution (5). These alterations in brain and behavior occur in young animals, months before the onset of these diseases (4, 22).
Most research on the neural substrates of the studied behaviors has focused on the amygdala and hippocampus. Because these (and other) brain areas have glucocorticoid receptors (25), the role stress plays in plasticity in these regions has received much attention. The amygdala is an assemblage of nuclei and has no reliable structural or functional definition (26). The traditional guiding concept has been that of a “chemosensory” amygdala with its divisions based on input from the main or olfactory amygdala (for processing odor cues) versus the accessory or vomeronasal amygdala (for processing pheromonal cues). The “extended” amygdala concept relates to a functionally integrated series of nuclei (27). Regardless of the definition used, amygdaloid nuclei are involved in activation of the hypothalamo–pituitary–adrenal (HPA) axis both via their role in the control of pituitary adrenocorticotropic hormone release by stressors and the integration of behavioral responses to fear and/or anxiety-provoking conditions (28). The hippocampus both receives and sends projections to the amygdaloid nuclei, including CA1 and CA3 (29–32). Traditionally, the hippocampus is thought to inhibit stress-induced activation of the HPA (28). Although the amygdala and hippocampus tend to be studied separately for their roles in emotional behaviors and learning and memory, respectively, both structures are involved (33, 34).
We find that this ancestral exposure promotes weight gain and, as such, provides pivotal empirical evidence that exposure to an endocrine disruptor in generations past results in substantial weight gain in the descendants. We also replicate the finding that this transgenerational epigenetic modification influences how individuals respond to events in their own life history (4, 22) as well as the work of others that CRS experience influences an individual’s physiological and behavioral phenotypes as an adult. CRS has an immediate and long-lasting effect on BW that correlates with CORT secretion later in life (35–38) as well as a negative relationship between stress and CORT and TESTO (39, 40). The behavioral tests measuring emotionality, anxiety, learning, and memory also reveal the well-established effects of CRS. We find a lack of effect of stress (or lineage) in the FS test (36, 41). CRS has a profound effect on the structure and function of the hippocampus (42), and, with a single exception in the CRTX, differential expression of apoptosis genes of >1.2 is restricted to the CA1 and CA3 (Fig. S3). GST (Gsp) genes are thought to be involved in stress-related oxidative damage in the pyramidal cells of the CA1 and CA3, and, in mice, CRS down-regulates Gsp in these regions (43). A similar down-regulation is observed in the present study in CA1 (but not CA3) in both C-L and V-L males. Thus, for some traits, lineage and CRS have effects independent of each other. Equally important, other traits, such as circulating levels of leptin and FS, show no evidence that either epigenetic modification has an effect. Finally, the fact that C-L and V-L individuals were housed together (dyad) yet responded differently further emphasizes that the transgenerational epigenetic modification fundamentally alters how the individual responds to a common challenge.
What is significant about this study is that ancestral exposure to an endocrine disruptor changes how individuals respond to CRS in adolescence. Landscape analyses reveal that, depending on the phenotype, different relationships emerge. For example, at all three levels (physiological, behavioral, and brain metabolism), the effects of lineage are most apparent in the stress condition, suggesting that V-L males respond differently after CRS than do C-L males. In the physiological landscape (Fig. 2A), TESTO and CORT are mostly responsive to the effect of lineage but only in the stress condition. In both lineages, CRS slows weight gain, yet the V-L nonstress males gain weight more rapidly. The most notable effects in the behavioral landscape are the reversed effects of stress in the OF between C-L and V-L males (Fig. 2B). C-L stress males show lower anxiety, defined by fewer entries and less time in the center arena, whereas animals not exposed to CRS show elevated anxiety. This effect is reversed in the V-L animals: Stressed males show higher levels of anxiety, which is consistent with the findings of Soc 1 and 2. In the nonstress condition, V-L males show a heightened state of anxiety compared with C-L males. In C-L males, there is little effect of CRS on anxiety, as defined by the willingness to explore novel social interactions. However, in V-L males, anxiety state is decreased after CRS even beyond that displayed by C-L males. The brain metabolism landscape (Fig. 2C) shows that the amygdaloid nuclei are differentially affected by lineage. For example, cytochrome oxidase activity in PMCo is higher in C-L males regardless of stress condition. The MePD shows opposite effects after CRS (pronounced increase in C-L males and decrease in V-L males). In the MeAmy, cytochrome oxidase activity is opposite in the lineages, depending on stress. We also see that the ST, and not the bed nucleus of the ST (BnST), is markedly changed, indicating that activity in this major pathway is being modified by lineage. In CA1 and CA3, stress decreases metabolic activity in the V-L males but has no such effect in C-L males.
Although the primary focus of the current study is a systems biology approach to understanding how the brain responds to ancestral exposures and environmental stress, a more targeted approach that considers specific genes can also provide insights into the general pathways and processes identified. Considering genes important in stress reactivity, V-L males have higher levels of BDNF (P = 0.03) in the BLA, whereas C-L males have higher levels of catechol-O-methyltransferase (COMT; P = 0.02) in CA1. The effects of CRS are observed in the BLA (COMT is higher in C-L nonstress males, P = 0.003) and the CA1 [melanocortin 4 receptor (Mc4r) is higher in the C-L stress group, P = 0.008]. Depending on the nucleus, gene expression can be negatively correlated: CRS in C-L animals increases expression of dopamine receptor D2 >1.5-fold in the BLA but decreases it by >1.5-fold in the CA3. The effects of the interaction of lineage and CRS are observed in the CA1 [cytochrome P450, family 19, subfamily A, polypeptide 1 (Cyp19a1) is lower in the V-L stress condition, P = 0.03], CA3 [melanocortin 3 receptor (Mc3r) and nuclear receptor subfamily 3, group C, member 1 (Nr3c1) are higher in the V-L stress group, P = 0.04 and 0.03, respectively], and CRTX [nuclear respiratory factor 1 (Nrf1) is higher in the V-L stress condition, P = 0.02]. However, in a genome-wide context, the reductionist approach and consideration of individual genes is not overly informative.
Further analysis of the systems biology of these coordinated phenomena used a more extensive molecular investigation. A bioinformatics analysis of the altered brain transcriptomes revealed gene networks associated with each brain region. These regional-specific gene networks provide a molecular basis for the physiological and behavioral alterations observed. Although the gene networks were distinct, many of the altered genes in the various regions were in similar signaling pathways. For example, the olfactory transduction pathway was affected by nearly all of the lineage and stress comparisons (Tables S3 and S4 and Fig. S6). An olfactory receptor promoter has been shown to have an epigenetic transgenerational alteration in sperm (6). CRS altered 17 genes in the CA1 of V-L males and in the CRTX of C-L males. Why should genes involved in olfaction be expressed in areas of the brain not involved with olfaction and taste? Olfactory and vomeronasal receptors as a group are among the most rapidly evolving of all genes and have been linked to higher processing centers in the brain as well as to behavior (44, 45). Such findings may indicate the neurobiological and neuromolecular basis of the Proust effect, a phenomenon in which an involuntary memory reaction is triggered by an olfactory input (46). This approach also identifies brain signaling pathways associated with neurodegenerative disease (Table S4). Thus, the altered comparative gene sets and gene networks identified are anticipated to be critical in the vinclozolin lineage and stress effects on the physiological and behavioral phenotypes observed.
How an ancestral environmental exposure modifies the germ-line epigenome and promotes epigenetic transgenerational inheritance is critical in any consideration of tissue function. The exposure to CRS during adolescence clearly influences subsequent brain development and behavior but is itself altered by ancestral exposures and epigenetic transgenerational inheritance. The molecular basis of this environmental influence on phenotype involves unique gene networks associated with these altered phenotypes. As such, it is one of the few systems biology approaches to link ancestral and context-dependent environmental factors to illustrate bringing “the phenotype into being” (2) down to the molecular basis of this phenomena.
Materials and Methods
Detailed materials and methods and corresponding references are presented in SI Materials and Methods. In brief, two different cohorts of male rats of the F3 generation of V-L and C-L produced at Washington State University were shipped to the University of Texas at Austin on the day after weaning (Fig. S1A). Rats were randomly pair-housed (one of each lineage) and remained in these dyads throughout the duration of the study. On the day after the last behavioral test, the animals were killed by rapid decapitation, and tissue and blood samples were taken within 3 min (Fig. S9A, A and B). Brain regions were isolated, and RNA for animals from different litters was pooled to generate three different pools with the highest biological variation possible to be used in the microarray analysis.
Supplementary Material
Acknowledgments
We thank R. Tracey, L. Walker, D. Walker, and Y. Matsumoto for technical assistance as well as H. Johnson for assistance in preparation of the manuscript. We thank Drs. A. Gore, C. Guerrero-Bosagna, H. Hofmann, and M. Montfils for critical input and comments on the manuscript. This work was supported in part by National Institutes of Health Grants ES 017538 (to D.C.) and ES 012974 (to M.K.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: All microarray CEL files reported in this study have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE26737).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1118514109/-/DCSupplemental.
References
- 1.Gilbert SF. Ecological developmental biology: Developmental biology meets the real world. Dev Biol. 2001;233(1):1–12. doi: 10.1006/dbio.2001.0210. [DOI] [PubMed] [Google Scholar]
- 2.Waddington CH. The epigenotype. Endeavour. 1942;1:18–20. [Google Scholar]
- 3.Guerrero-Bosagna CM, Skinner MK. Epigenetic transgenerational effects of endocrine disruptors on male reproduction. Semin Reprod Med. 2009;27:403–408. doi: 10.1055/s-0029-1237428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skinner MK, Anway MD, Savenkova MI, Gore AC, Crews D. Transgenerational epigenetic programming of the brain transcriptome and anxiety behavior. PLoS ONE. 2008;3:e3745. doi: 10.1371/journal.pone.0003745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guerrero-Bosagna C, Settles M, Lucker B, Skinner MK. Epigenetic transgenerational actions of vinclozolin on promoter regions of the sperm epigenome. PLoS ONE. 2010;5:e13100. doi: 10.1371/journal.pone.0013100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skinner MK, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol Metab. 2010;21(4):214–222. doi: 10.1016/j.tem.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crews D. Epigenetics and its implications for behavioral neuroendocrinology. Front Neuroendocrinol. 2008;29:344–357. doi: 10.1016/j.yfrne.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCormick CM, Mathews IZ. Adolescent development, hypothalamic-pituitary-adrenal function, and programming of adult learning and memory. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:756–765. doi: 10.1016/j.pnpbp.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Conrad CD. A critical review of chronic stress effects on spatial learning and memory. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:742–755. doi: 10.1016/j.pnpbp.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Romeo RD, McEwen BS. Stress and the adolescent brain. Ann N Y Acad Sci. 2006;1094:202–214. doi: 10.1196/annals.1376.022. [DOI] [PubMed] [Google Scholar]
- 12.Romeo RD. Adolescence: A central event in shaping stress reactivity. Dev Psychobiol. 2010;52:244–253. doi: 10.1002/dev.20437. [DOI] [PubMed] [Google Scholar]
- 13.Romeo RD, Tang AC, Sullivan RM. Early life experiences: Enduring behavioral, neurological, and endocrinological consequences. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. 2nd Ed. New York: Academic; 2009. pp. 1975–2006. [Google Scholar]
- 14.McCormick CM, Mathews IZ, Thomas C, Waters P. Investigations of HPA function and the enduring consequences of stressors in adolescence in animal models. Brain Cogn. 2010;72:73–85. doi: 10.1016/j.bandc.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 15.McEwen BS. Stress, sex, and neural adaptation to a changing environment: Mechanisms of neuronal remodeling. Ann N Y Acad Sci. 2010;1204(Suppl):E38–E59. doi: 10.1111/j.1749-6632.2010.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scarpino S, Gillette R, Crews D. MultiDimBio: An R package for the functional landscape analysis of multivariate data. J Stat Softw. 2012 in press. [Google Scholar]
- 17.Weiss P. Principles of Development. New York: Holt; 1939. [Google Scholar]
- 18.Novikoff AB. The concept of integrative levels and biology. Science. 1945;101(2618):209–215. doi: 10.1126/science.101.2618.209. [DOI] [PubMed] [Google Scholar]
- 19.Kitano H. Systems biology: A brief overview. Science. 2002;295:1662–1664. doi: 10.1126/science.1069492. [DOI] [PubMed] [Google Scholar]
- 20.DeVries AC, Craft TK, Glasper ER, Neigh GN, Alexander JK. 2006 Curt P. Richter award winner: Social influences on stress responses and health. Psychoneuroendocrinology. 2007;32:587–603. doi: 10.1016/j.psyneuen.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Wood GE, Young LT, Reagan LP, McEwen BS. Acute and chronic restraint stress alter the incidence of social conflict in male rats. Horm Behav. 2003;43(1):205–213. doi: 10.1016/s0018-506x(02)00026-0. [DOI] [PubMed] [Google Scholar]
- 22.Crews D, et al. Transgenerational epigenetic imprints on mate preference. Proc Natl Acad Sci USA. 2007;104:5942–5946. doi: 10.1073/pnas.0610410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crews D, Rushworth D, Gonzalez-Lima F, Ogawa S. Litter environment affects behavior and brain metabolic activity of adult knockout mice. Front Behav Neurosci. 2009;3:12. doi: 10.3389/neuro.08.012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nilsson EE, et al. Gene bionetwork analysis of ovarian primordial follicle development. PLoS ONE. 2010;5:e11637. doi: 10.1371/journal.pone.0011637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morimoto M, Morita N, Ozawa H, Yokoyama K, Kawata M. Distribution of glucocorticoid receptor immunoreactivity and mRNA in the rat brain: An immunohistochemical and in situ hybridization study. Neurosci Res. 1996;26(3):235–269. doi: 10.1016/s0168-0102(96)01105-4. [DOI] [PubMed] [Google Scholar]
- 26.Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- 27.Alheid GF. Extended amygdala and basal forebrain. Ann N Y Acad Sci. 2003;985:185–205. doi: 10.1111/j.1749-6632.2003.tb07082.x. [DOI] [PubMed] [Google Scholar]
- 28.Herman JP, et al. Central mechanisms of stress integration: Hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24(3):151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Krettek JE, Price JL. Projections from the amygdaloid complex and adjacent olfactory structures to the entorhinal cortex and to the subiculum in the rat and cat. J Comp Neurol. 1977;172:723–752. doi: 10.1002/cne.901720409. [DOI] [PubMed] [Google Scholar]
- 30.Pikkarainen M, Rönkkö S, Savander V, Insausti R, Pitkänen A. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. J Comp Neurol. 1999;403(2):229–260. [PubMed] [Google Scholar]
- 31.Ishikawa A, Nakamura S. Ventral hippocampal neurons project axons simultaneously to the medial prefrontal cortex and amygdala in the rat. J Neurophysiol. 2006;96:2134–2138. doi: 10.1152/jn.00069.2006. [DOI] [PubMed] [Google Scholar]
- 32.Kishi T, Tsumori T, Yokota S, Yasui Y. Topographical projection from the hippocampal formation to the amygdala: A combined anterograde and retrograde tracing study in the rat. J Comp Neurol. 2006;496:349–368. doi: 10.1002/cne.20919. [DOI] [PubMed] [Google Scholar]
- 33.Packard MG, Teather LA. Amygdala modulation of multiple memory systems: Hippocampus and caudate-putamen. Neurobiol Learn Mem. 1998;69(2):163–203. doi: 10.1006/nlme.1997.3815. [DOI] [PubMed] [Google Scholar]
- 34.Hale MW, et al. Exposure to an open-field arena increases c-Fos expression in a distributed anxiety-related system projecting to the basolateral amygdaloid complex. Neuroscience. 2008;155:659–672. doi: 10.1016/j.neuroscience.2008.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barha CK, Brummelte S, Lieblich SE, Galea LA. Chronic restraint stress in adolescence differentially influences hypothalamic-pituitary-adrenal axis function and adult hippocampal neurogenesis in male and female rats. Hippocampus. 2011;21:1216–1227. doi: 10.1002/hipo.20829. [DOI] [PubMed] [Google Scholar]
- 36.Ulloa JL, et al. Comparison of the antidepressant sertraline on differential depression-like behaviors elicited by restraint stress and repeated corticosterone administration. Pharmacol Biochem Behav. 2010;97(2):213–221. doi: 10.1016/j.pbb.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Harris RB, Palmondon J, Leshin S, Flatt WP, Richard D. Chronic disruption of body weight but not of stress peptides or receptors in rats exposed to repeated restraint stress. Horm Behav. 2006;49:615–625. doi: 10.1016/j.yhbeh.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 38.Solomon MB, Jones K, Packard BA, Herman JP. The medial amygdala modulates body weight but not neuroendocrine responses to chronic stress. J Neuroendocrinol. 2010;22(1):13–23. doi: 10.1111/j.1365-2826.2009.01933.x. [DOI] [PubMed] [Google Scholar]
- 39.Hardy MP, et al. Stress hormone and male reproductive function. Cell Tissue Res. 2005;322(1):147–153. doi: 10.1007/s00441-005-0006-2. [DOI] [PubMed] [Google Scholar]
- 40.Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. J Neuroendocrinol. 2002;14:506–513. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- 41.Gregus A, Wintink AJ, Davis AC, Kalynchuk LE. Effect of repeated corticosterone injections and restraint stress on anxiety and depression-like behavior in male rats. Behav Brain Res. 2005;156(1):105–114. doi: 10.1016/j.bbr.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 42.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 43.Ejchel-Cohen TF, et al. Chronic restraint stress decreases the expression of glutathione S-transferase pi2 in the mouse hippocampus. Brain Res. 2006;1090(1):156–162. doi: 10.1016/j.brainres.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 44.Feldmesser E, et al. Widespread ectopic expression of olfactory receptor genes. BMC Genomics. 2006;7:121. doi: 10.1186/1471-2164-7-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dulac C, Wagner S. Genetic analysis of brain circuits underlying pheromone signaling. Annu Rev Genet. 2006;40:449–467. doi: 10.1146/annurev.genet.39.073003.093937. [DOI] [PubMed] [Google Scholar]
- 46.Chu S, Downes JJ. Odour-evoked autobiographical memories: Psychological investigations of Proustian phenomena. Chem Senses. 2000;25(1):111–116. doi: 10.1093/chemse/25.1.111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.