NEUROSCIENCE Correction for “Anterograde or retrograde transsynaptic labeling of CNS neurons with vesicular stomatitis virus vectors,” by Kevin T. Beier, Arpiar Saunders, Ian A. Oldenburg, Kazunari Miyamichi, Nazia Akhtar, Liqun Luo, Sean P. J. Whelan, Bernardo Sabatini, and Constance L. Cepko, which appeared in issue 37, September 13, 2011, of Proc Natl Acad Sci USA (108:15414–15419; first published August 8, 2011; 10.1073/pnas.1110854108).
The authors note the following: “Shortly after publication of the above manuscript, the replication-competent virus stock, vesicular stomatitis virus (VSV) (LCMV-G), used for the experiments in the paper, was found to be contaminated with wild type VSV [referred to as VSV (VSV-G)]. In addition, the replication-incompetent stock, VSV ΔG (LCMV-G) stock, only used for the data in Fig. 3B, was also contaminated by the same wild type VSV. When a pure stock of VSV (LCMV-G) was made, it was found to give inefficient anterograde transmission, in contrast to the efficient anterograde transmission seen with the mixed stock. Most cells infected with the pure VSV (LCMV-G) stock at an initial inoculation site were glia, although the virus did transmit anterogradely to a small number of neurons. The original VSV (VSV-G) stock used for the experiments published in the paper, which contaminated the VSV (LCMV-G) stock, was found to give efficient and specific anterograde transmission. The anterograde transmission was for all injections made directly into the brain, including from the caudate putamen to all of the anterograde target locations published in our paper. Moreover, it did not give retrograde labeling (e.g., see Fig. 2A). However, neither the original VSV (VSV-G) stock, nor an independent VSV (VSV-G) stock obtained from another lab, were found to give anterograde tracing from the eye, or from the nose, to the brain, when inoculated into either of these peripheral locations. The anterograde tracing from the eye or nose to the brain was only seen in our initial studies using the mixed stock, and in our repeated set of experiments with this same mixed stock. We have since plaque purified viruses from this mixed stock. We tested 21 plaque purified viruses for their ability to give anterograde tracing from a peripheral injection site by injecting into the eye and examining the brains. Several stocks from plaque-purified viruses gave such anterograde transmission, and all of these viruses encoded VSV-G only (i.e., not LCMV-G) (for an example, see Fig. A). These same stocks also give the anterograde tracing patterns seen from inoculations of the caudate-putamen (similar to patterns shown in Fig. 3). We are now studying these VSV-G viruses to determine why they are such effective anterograde tracers, and why individual stocks differ when injected into peripheral sites. The differences may be due to the fact that injecting a peripheral site demands long distance travel from the initially infected cells to the brain, or it may have more to do with some other aspect of the sites being peripheral to the brain.
Fig. A.
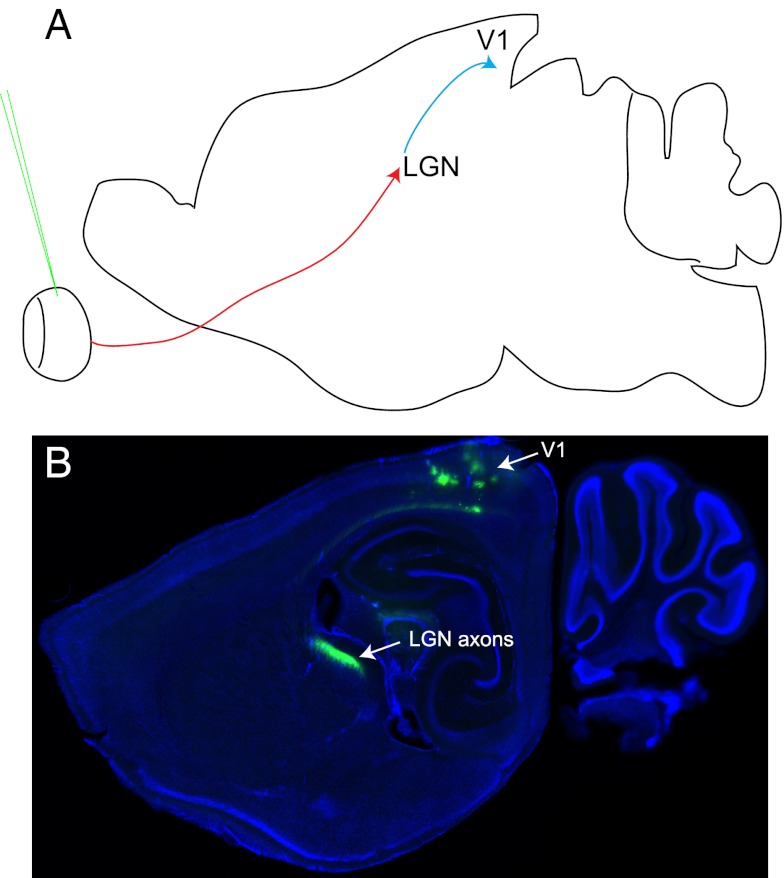
Anterograde pattern of spread of VSV (VSV-G) from a virus stock derived from a purified plaque. Anterograde transmission was tested by injection into the vitreous body of the eye. (A) A diagram of a parasaggital section of the brain illustrates the expected pattern of transmission for an anterograde transsynaptic virus injected into the eye. It would be expected to label several brain centers involved in visual processing, including the lateral geniculate nucleus (LGN) and visual cortex area 1 (V1). The red arrow indicates the path of the retinal ganglion cell axons to their direct targets, the cells of the LGN. The blue arrow indicates the path of the LGN axons to their V1 targets. The green needle indicates the injection site. (B) A parasaggital section from an injected brain at 7 d post infection, showing labeling of the LGN and V1. All injections that gave brain labeling using this virus stock showed a similar pattern (5/10 animals injected).
“For those neuroscientists wishing to perform anterograde, polysynaptic tracing, we recommend using the VSV (VSV-G) stock that we have plaque purified. For monosynaptic tracing, the ΔG VSV genome can be used with any of the viral G proteins published in PNAS: VSV-G (for anterograde), LCMV-G (for anterograde), or RABV-G (for retrograde). It may be the case that VSV-G is more efficient for anterograde monosynaptic tracing than LCMV-G. More testing needs to be done, particularly in vivo, to determine the relative efficiencies of these G proteins for monosynaptic tracing.
“We stand by the conclusions of the paper that VSV is an effective transsynaptic tracer, and that the G protein determines the direction of transmission. The RABV-G gives retrograde transmission, while the VSV-G and the LCMV-G direct anterograde transmission, with the VSV-G giving more efficient transmission than LCMV-G as a replication-competent virus.”