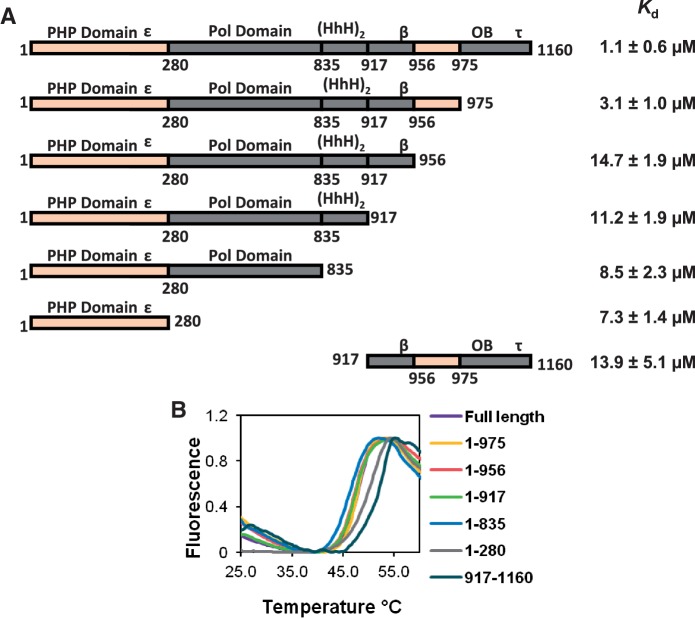
Figure 3.
UmuD binds α at two distinct binding sites. (A) Kd values for UmuD binding to various α truncations. The large difference between the Kd values for the truncations α1–975 and α1–956 indicates that one binding site is between residues 956–975. The reduced but still significant binding constants for the truncations α1–956, α1–917, α1–835 and α1–280 suggests another UmuD binding site is present in the N-terminal domain α1–280. The domains of α are labeled above the schematic of the protein; ε, β and τ indicate the location of the respective binding site for each protein, HhH indicates the helix-hairpin-helix domain involved in double-stranded DNA binding, OB indicates the OB-fold domain involved in single-stranded DNA binding. (B) Melting curves for the various α truncations used show comparable stability to that of full-length α.