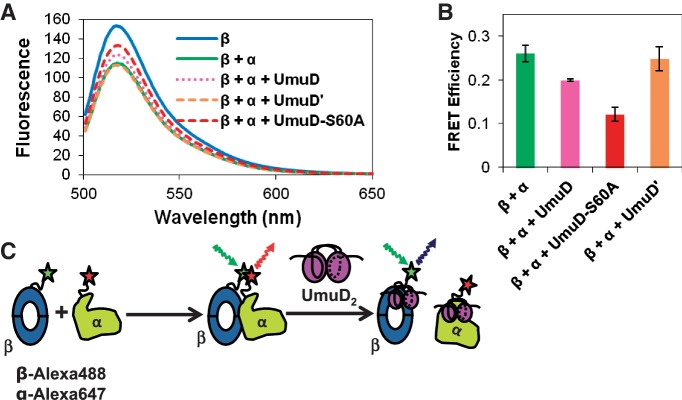
Figure 7.
Fluorescence resonance energy transfer analysis shows that UmuD disrupts the interaction between the α polymerase, labeled with Alexa Fluor 647 C2-maleimide, and the β processivity clamp, labeled with Alexa Fluor 488 C5-maleimide. (A) FRET was reduced in the presence of UmuD and UmuD-S60A but not in the presence of UmuD′. FRET between β and α was reduced in the presence of UmuD-S60A in a concentration-dependent manner, from 1 to 40 μM UmuD-S60A (not shown). (B) Bar graph showing FRET efficiency at each condition; representative of five trials. The error bars represent the standard deviation of at least three trials. (C) Schematic of FRET experiment showing that the FRET observed upon interaction of β and α is reduced with the addition of full-length UmuD, but not UmuD'. As UmuD interacts with both α and β at competing sites, the α–β interaction could be disrupted by interaction of UmuD with either protein. Other modes of interaction are possible, in which UmuD induces a conformational change in one or both proteins that inhibits binding, for example, but since UmuD interacts with α at the same site where β interacts with α and UmuD interacts with β at a site overlapping where α interacts with β (69), direct competition is the most straightforward model.