Background
In July 2010, the Medical Advisory Secretariat (MAS) began work on a Chronic Obstructive Pulmonary Disease (COPD) evidentiary framework, an evidence-based review of the literature surrounding treatment strategies for patients with COPD. This project emerged from a request by the Health System Strategy Division of the Ministry of Health and Long-Term Care that MAS provide them with an evidentiary platform on the effectiveness and cost-effectiveness of COPD interventions.
After an initial review of health technology assessments and systematic reviews of COPD literature, and consultation with experts, MAS identified the following topics for analysis: vaccinations (influenza and pneumococcal), smoking cessation, multidisciplinary care, pulmonary rehabilitation, long-term oxygen therapy, noninvasive positive pressure ventilation for acute and chronic respiratory failure, hospital-at-home for acute exacerbations of COPD, and telehealth (including telemonitoring and telephone support). Evidence-based analyses were prepared for each of these topics. For each technology, an economic analysis was also completed where appropriate. In addition, a review of the qualitative literature on patient, caregiver, and provider perspectives on living and dying with COPD was conducted, as were reviews of the qualitative literature on each of the technologies included in these analyses.
The Chronic Obstructive Pulmonary Disease Mega-Analysis series is made up of the following reports, which can be publicly accessed at the MAS website at: http://www.hqontario.ca/en/mas/mas_ohtas_mn.html.
Chronic Obstructive Pulmonary Disease (COPD) Evidentiary Framework
Influenza and Pneumococcal Vaccinations for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Smoking Cessation for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Community-Based Multidisciplinary Care for Patients With Stable Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Pulmonary Rehabilitation for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Long-term Oxygen Therapy for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Noninvasive Positive Pressure Ventilation for Acute Respiratory Failure Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Noninvasive Positive Pressure Ventilation for Chronic Respiratory Failure Patients With Stable Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Hospital-at-Home Programs for Patients With Acute Exacerbations of Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Home Telehealth for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Cost-Effectiveness of Interventions for Chronic Obstructive Pulmonary Disease Using an Ontario Policy Model
Experiences of Living and Dying With COPD: A Systematic Review and Synthesis of the Qualitative Empirical Literature
For more information on the qualitative review, please contact Mita Giacomini at: http://fhs.mcmaster.ca/ceb/faculty_member_giacomini.htm.
For more information on the economic analysis, please visit the PATH website: http://www.path-hta.ca/About-Us/Contact-Us.aspx.
The Toronto Health Economics and Technology Assessment (THETA) collaborative has produced an associated report on patient preference for mechanical ventilation. For more information, please visit the THETA website: http://theta.utoronto.ca/static/contact.
Objective
The objective of this report series is to create an evidentiary base and economic analysis that will guide investment in the treatment of chronic obstructive pulmonary disease (COPD) in a way that optimizes patient outcomes and system efficiencies. This evidentiary platform concerning the effectiveness and cost-effectiveness of treatment strategies for patients with COPD will be used to build a provincial COPD strategy.
Clinical Need and Target Population
COPD is a disease state characterized by airflow limitation that is not fully reversible. The airflow limitation is usually both progressive and associated with an abnormal inflammatory response by the lungs to noxious particles or gases. (1;2) The airflow limitation is caused by small airway disease (obstructive bronchiolitis) and parenchymal destruction (emphysema), both of which contribute to the disease to varying degrees, depending on the person. Chronic inflammation causes structural changes in the lungs and narrowing of the small airways. Inflammatory processes also cause destruction of the lung parenchyma, which leads to the loss of alveolar attachments to the small airways and decreases lung elastic recoil. These changes diminish the ability of the airways to remain open during expiration. (1)
The most common symptoms of COPD include chronic and progressive breathlessness, cough, sputum production, wheezing, and chest congestion. (1;3) In addition to the airflow restriction and changes to the lung, COPD is associated with systemic effects and comorbidities. (1;2) Systemic effects include weight loss, nutritional abnormalities and malnutrition, and skeletal muscle dysfunction. (1) Common comorbidities are ischemic heart disease, osteoporosis, respiratory infection, bone fractures, depression and anxiety, diabetes, sleep disorders, anemia, glaucoma and cataracts, and cancer. (1;2)
Natural History of COPD
COPD is a progressive disease. The rate of progression varies and may occur over several years or several decades, depending on factors such as continued exposure to noxious particles (e.g., tobacco smoke). (1;3) There are several systems for classifying the severity of COPD; one of the most widely used is the Global Initiative for Chronic Obstructive Lung Disease (GOLD) staging criteria, which are based on postbronchodilator spirometry (forced expiratory volume in 1 second [FEV1]). In the GOLD system there are 4 stages, which range from mild to very severe (Table 1). (1)
Table 1: GOLD Staging Criteria for COPD*.
| Stage | Severity | FEV1/FVC | FEV1 | Symptoms |
|---|---|---|---|---|
| I | Mild | < 0.70 | FEV1 ≥ 80% predicted | Symptoms may or may not be present Possible symptoms include chronic cough and sputum production |
| II | Moderate | < 0.70 | 50% ≤ FEV1 < 80% predicted | Shortness of breath on exertion Cough and sputum production are sometimes present |
| III | Severe | < 0.70 | 30% ≤ FEV1 < 50% predicted | Greater shortness of breath, reduced exercise capacity, fatigue, and repeated exacerbations |
| IV | Very severe | < 0.70 | FEV1 < 30% predicted or FEV1 < 50% predicted plus chronic respiratory failure | Respiratory failure, which may also lead to cor pulmonale |
Abbreviations: COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
Source: Global Initiative for Chronic Obstructive Lung Disease, 2010. (1)
The disease course varies, but typically patients fluctuate between stable disease and acute exacerbations, which become more common as the disease progresses. Acute exacerbations are periods when symptoms worsen. There is debate about the best definition for exacerbations; a consensus definition developed by GOLD defines an acute exacerbation as “an event in the natural course of the disease characterized by a change in the patient’s baseline dyspnea, cough, and/or sputum that is beyond normal day-to-day variations, is acute in onset, and may warrant a change in regular medication.” (1) Patients may also experience a variety of other symptoms, such as worsening exercise tolerance, fatigue, malaise, and decreased oxygen saturation. (4) After an acute exacerbation, the individual may not recover to his/her previous level of airflow limitation, and this permanent loss of lung function contributes to the progressive nature of the disease. (3)
Two-thirds of exacerbations are caused by either an infection of the tracheobronchial tree or air pollution, but the cause is unknown in the remaining cases. (1;3) Risk factors for exacerbations include disease severity, winter months, and a previous exacerbation in the past 8 weeks. (4;5) The frequency of exacerbations varies by disease severity. Using data from the ISOLDE Study, the European Respiratory Society Study on COPD, and the Copenhagen City Lung Study, Donaldson et al (4) found that patients with severe disease (GOLD stage III) experienced an average of 3.43 exacerbations per year, while patients with moderate disease (GOLD stage II) experienced an average of 2.68 exacerbations per year.
Epidemiology of COPD
Prevalence
Estimates of COPD prevalence vary depending on the methods and diagnostic criteria used to identify cases. Many of the prevalence estimates are also believed to be underestimates due to underdiagnosis and underrecognition of COPD and to limited diagnoses of mild cases, as individuals often do not require health care services until they reach the moderate to severe stages of the disease. (1;6)
Based on the Canadian Community Health Survey, in 2007 about 4.4% of Canadians self-reported that they had been diagnosed with COPD by physicians. (7) Based on Ontario administrative data sets, Gershon et al (8) estimated the 2007 age- and sex-standardized prevalence of COPD in Ontario to be 9.5%. The prevalence of COPD has increased over time; Gershon et al (8) found a 23% increase in the prevalence rate between 1996 and 2007 (1996, 7.8%; 2007, 9.5%), and this corresponds to an increase of 64.8% in the number of adults with COPD. The aging population alone does not entirely account for this increase. (8)
Gershon et al (8) also found the prevalence of COPD to be higher in men than in women: in 2007, the age- and sex-standardized prevalence was 9.0% in women and 10.3% in men; however, prevalence is increasing faster in women than in men, with a 33.4% increase in the age-standardized prevalence rate in women, compared with a 12.9% increase in men (P < 0.001). Prevalence also varies by age group, as shown in Figure 1.
Figure 1: Prevalence of COPD in Ontario in 2006/2007 (Adults Aged 35 Years and Older)*.
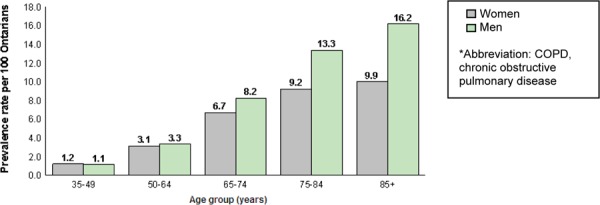
Source: Institute for Clinical Evaluative Sciences, 2011. (9)
Incidence
Based on Ontario administrative data sets, the 2007 age- and sex-standardized incidence of COPD in Ontario was 8.5 cases per 1,000 adults. (8) Gershon et al (8) showed that the incidence rate has been declining since 1996, when it was 11.8 cases per 1,000 adults. The age-standardized incidence rate is higher in males than in females (9.4 cases per 1,000 adults vs. 7.8 cases per 1,000 adults, respectively); however, the incidence rate has been declining faster in males than females (% decline since 1996, 32.3% vs. 24.7%, respectively). (8)
Risk Factors for COPD
The most common risk factor for COPD—and the primary cause of COPD in 80% to 90% of cases—is exposure to tobacco smoke. (7) There are numerous other risk factors, however, including exposure to occupational dusts and chemicals (including vapours, irritants, and fumes), indoor air pollution (e.g., from burning biomass fuels for heating and cooking in confined spaces in developing countries), outdoor air pollution, genetics, lung growth and development, oxidative stress, respiratory infections and previous tuberculosis, and asthma. The quality and strength of evidence supporting these risk factors vary, with the strongest evidence being for tobacco smoke, occupational exposures, indoor air pollution, and alpha1-antitrypsin deficiencies. (1;10;11)
Diagnosis of COPD
The GOLD guidelines recommend that any individual with breathlessness, chronic cough, or sputum production—especially those with risk factors (such as cigarette smokers)—be evaluated for COPD. (1) Spirometry, the best standardized, objective measurement for airflow limitation, should be used to confirm all COPD diagnoses. (12) Spirometry (or pulmonary function tests) include the forced vital capacity (FVC, volume of air forcibly exhaled from the point of maximal inspiration) and the FEV1 (volume of air exhaled during the first second of the FVC measurement). (1) During a test, patients breathe into a mouthpiece attached to a spirometer. The results are compared with standard scores; with reference values based on age, height, sex, and race; and with results presented as a percentage of the predicted value. (1)
Apart from spirometry, other tests may be conducted to help assess severity of disease and provide additional information necessary for treatment. These tests include bronchodilator reversibility testing, chest x-ray, and arterial blood gas measurements. (1;12)
Both over- and underdiagnosis of COPD are possible issues. Overdiagnosis can occur when the diagnosis is based solely upon an individual’s medical history and physical examination and is not confirmed by spirometry. (3) Underdiagnosis can occur due to underrecognition of COPD by both clinicians and patients. (1;6)
Management of COPD
COPD management and treatment is a staged process depending on the severity of the disease, with new treatments/management strategies introduced as needed. It begins with avoiding risk factors (e.g., vaccinations, smoking cessation, etc.), and as the disease progresses, introducing additional treatments and medications (e.g., drug therapy, pulmonary rehabilitation, oxygen therapy, etc.). (1;2) More detailed information regarding many of these treatment and management strategies is provided in this report.
Impact of COPD
First and foremost, COPD has a considerable impact on the person with the disease. This impact varies and is influenced not just by the degree of airflow limitation, but also by the severity of symptoms, including breathlessness, decreased exercise capacity, systemic effects, and comorbidities. (1) These symptoms can have a substantial impact on people living with the disease: based on the 1998/1999 National Population Health Survey, 51% of Canadians with COPD reported that their disease restricted their activity at home, at work, or in other activities. (13) In addition, people with moderate to severe COPD typically experience 1 or more acute exacerbations per year. These exacerbations impact health-related quality of life (HRQOL) and lung function; may require hospitalization and invasive treatment such as invasive mechanical ventilation (IMV); and increase the risk of mortality.
COPD is the fourth leading cause of death in Canada and is expected to be the third leading cause of death by 2020. (14;15) The 2007 age- and sex-standardized mortality rate1 in Ontario was 4.3%, which translates to 32,156 deaths. (8)
Apart from its impact on individual patients, COPD has a substantial effect on the health system. COPD is a leading cause of health care utilization, both globally and in Canada. In 1997, COPD was the fourth most common cause of hospitalization among Canadian men and the sixth most common among Canadian women. (13) The age- and sex-standardized average hospitalization rate from 1996 to 1999 was 632 hospitalizations per 100,000 adults in Ontario. (13) Furthermore, acute exacerbations of COPD are a leading cause of emergency department (ED) visits and hospitalizations, particularly in the winter.
The economic burden of COPD is high. The Canadian component of a large-scale international survey, Confronting COPD in North America and Europe, showed an annual direct cost of almost $2,000 (Cdn) per patient for COPD-related primary and secondary care visits, treatment, and laboratory tests. When combined with indirect costs accounting for lost productivity, the total annual cost was $3,195.52 (Cdn) per patient. (6) Of the direct costs, 60% were accounted for by unscheduled care visits, including primary care provider or specialist visits, hospitalizations, and ED visits. (6) Several studies in the United States have shown that per capita spending for COPD-related illness or patients with COPD are 2.5 to 2.7 times higher than for those without COPD. (1)
Project Scope
Technologies Under Review
After an initial review of health technology assessments (HTAs) and systematic reviews about COPD and consultations with experts in Ontario, the following COPD treatment strategies were selected for review: vaccinations, smoking cessation, community-based multidisciplinary care, pulmonary rehabilitation, long-term oxygen therapy (LTOT), noninvasive positive pressure ventilation (NPPV), hospital-at-home for the treatment of acute exacerbations of COPD, and home telehealth. In addition, a review of the qualitative literature examined patient, caregiver, and health care provider perspectives on living and dying with COPD.
Influenza and Pneumococcal Vaccinations
Similar to other chronic diseases, people with COPD are at increased risk of contracting both influenza and pneumonia. Both influenza and pneumonia can lead to acute exacerbations of COPD, a major cause of morbidity and mortality in COPD patients. Influenza and pneumococcal vaccinations may decrease the risk of infections and subsequent acute exacerbations in COPD patients.
Smoking Cessation
Tobacco smoke is the main risk factor for COPD and COPD-associated morbidity. Smoking cessation is the process of discontinuing the practice of inhaling a smoked substance. Smoking cessation strategies include both pharmacological and nonpharmacological (behavioural or psychosocial) approaches. The basic components of smoking cessation interventions include simple advice, written self-help materials, individual and group behavioural support, telephone quitlines, nicotine replacement therapy (NRT), and antidepressants. Since addiction to nicotine is a chronic relapsing condition that usually requires several attempts to achieve success, cessation support is usually tailored to individual needs with the recognition that, in general, the more intensive the support the greater the chance of success.
Community-Based Multidisciplinary Care
The term multidisciplinary refers to multiple disciplines on a care team, and the term interdisciplinary refers to multidisciplinary teams functioning in a coordinated and collaborative manner. There is consensus that a group of multidisciplinary professionals is necessary for optimum specialist management of a chronic illness. However, there is little evidence to guide the decision as to which professionals might be needed to optimize a multidisciplinary team.
Pulmonary Rehabilitation
Pulmonary rehabilitation refers to a multidisciplinary program of care for patients with chronic respiratory impairment that is individually tailored and designed to optimize physical and social performance and autonomy. Pulmonary rehabilitation is recommended as the standard of care for treating and rehabilitating patients with COPD who remain symptomatic despite treatment with bronchodilators.
Exercise training is the cornerstone of pulmonary rehabilitation programs and may include both aerobic and strength training. Other components may include psychological support, patient education, nutritional counselling, occupational therapy, medication information, and smoking cessation.
Long-Term Oxygen Therapy
Patients with severe or very severe COPD may also experience hypoxemia (low blood oxygen levels). Severe hypoxemia is defined as partial pressure of oxygen (PaO2) in arterial blood of less than or equal to 55 mm Hg; moderate hypoxemia is defined as a PaO2 between 56 mm Hg and 65 mm Hg. (16) Oxygen is a treatment option for COPD patients with hypoxemia because these individuals may have difficulty obtaining sufficient oxygen from the air, and providing oxygen corrects the deficiency of oxygen in arterial blood and prevents tissue hypoxia. LTOT is the extended use of oxygen for 15 hours per day or more. Potential safety concerns include accelerating a fire source such as a lit cigarette, falling over tubing, underusing oxygen, and patients with type 2 respiratory failure using high doses of oxygen, which would further elevate their tissue carbon dioxide levels.
Noninvasive Positive Pressure Ventilation
Respiratory failure occurs when the respiratory system cannot oxygenate the blood and/or remove carbon dioxide from the blood. NPPV can be used to treat both acute hypercapnic respiratory failure secondary to acute exacerbations of COPD and chronic respiratory failure in patients with severe COPD. NPPV provides ventilatory support through a facial or nasal mask and reduces inspiratory work. NPPV can often be used intermittently for short periods of time to treat respiratory failure, which allows patients to continue to eat, drink, talk, and participate in their own treatment decisions. In addition, patients do not require sedation, airway defence mechanisms and swallowing functions are maintained, trauma to the trachea and larynx are avoided, and the risk of ventilator-associated pneumonia (VAP) is reduced. Common complications are damage to facial and nasal skin, higher incidence of gastric distension with aspiration risk, sleeping disorders, and conjunctivitis. In addition, NPPV does not allow direct access to the airway to drain secretions, requires patients to cooperate, and has the potential to cause discomfort; compliance and tolerance may be low.
In addition to treating acute and chronic respiratory failure, NPPV can be used to wean patients from IMV through the gradual removal of ventilation support until the patient can breathe spontaneously. Finally, it has been proposed that NPPV can help prevent extubation failure by preventing the recurrence of acute respiratory failure after extubation and/or to treat respiratory failure when it recurs, thereby avoiding the need for reintubation.
Hospital-at-Home Programs
Hospital-at-home programs are services that provide patients with active treatment by health care professionals in the patient’s home for a condition that otherwise would require short-term acute hospital inpatient care. In general, when patients are enrolled in hospital-at-home programs for COPD exacerbations, they receive home visits by specialist nurses who monitor their symptoms, alter their treatment plans if needed, and in some programs, provide additional care such as pulmonary rehabilitation, patient and caregiver education, smoking cessation counselling, and support services. Patients remain the legal and medical responsibility of the hospital while being treated at home. The alternative to hospital-at-home programs for these patients is inpatient hospital care.
Home Telehealth
Given the chronic nature of COPD and the need for continuous patient management, home telehealth technologies are increasingly being used to treat outpatients. This review evaluated 2 types of telehealth used for COPD patients: home telemonitoring and telephone-only support.
Home telemonitoring is defined as the use of medical devices to remotely collect a patient’s vital signs and/or other biological health data and transmit these data to a monitoring station for a health care provider to interpret and respond to. Telephone-only support is disease management support given by a health care provider to a patient in his/her residence via telephone or videoconferencing technology, without transmitting patient biological data. There are 4 broad functions of home telehealth interventions for COPD patients:
to monitor vital signs or biological health data (e.g., oxygen saturation)
to monitor symptoms, medication, or other nonbiological endpoints (e.g., exercise adherence)
to provide information, education, and/or other support services (such as reminders to exercise or positive reinforcement)
to establish a communication link between patient and health care provider
Technologies Not Reviewed
A number of important technologies related to COPD were not included in this review. These include COPD prevention (see previous Medical Advisory Secretariat review on smoking cessation in the general population (17)), screening/early detection, drugs, and surgical interventions. A comprehensive provincial framework on COPD must also take these important topics into consideration.
COPD Prevention
Although the scope of the current project did not include prevention, one of the most important components of COPD prevention is smoking cessation. A 2010 Medical Advisory Secretariat review examined smoking cessation in the general population and provides information on the most effective strategies. The full report is available at: http://www.hqontario.ca/en/mas/mas_ohtas_tech_smoking_20100120.html (17)
The following points are key findings from this analysis:
The evidence suggests that pharmacotherapy, physician advice to quit, nursing interventions, hospital-based interventions, and proactive telephone counselling are effective and cost-effective in the short term.
There is poor quality data around other population-based smoking cessation strategies, including mass media campaigns, community interventions, quit-and-win contests, access to a quitline, and interventions for university and college campuses, making evaluation of their effectiveness and cost-effectiveness difficult.
Based on pooled summary estimates of effect and safety data, the most effective strategies are taking varenicline or bupropion or using NRTs, followed by physician advice to quit and nursing interventions in nonhospitalized smokers without cardiovascular disease. (17)
Apart from smoking, other risk factors for COPD include indoor (e.g., second-hand smoke) and outdoor air pollutants and occupational exposures to dust, vapours, and fumes. (10;11) COPD prevention initiatives should take these additional risk factors into consideration.
Screening/Early Detection of COPD
Underdiagnosis of COPD
People with known risk factors for COPD, such as smoking, are potential targets for screening and early intervention, and yet COPD is commonly believed to be underdiagnosed. Based on the Canadian Community Health Survey, in 2007 about 4.4% of Canadians self-reported having been diagnosed with COPD. (7) However, studies have shown that this figure is an underestimate of the true prevalence of COPD. For example, the Burden of Lung Disease (BOLD) study conducted spirometry testing (the reference standard for diagnosis of COPD) on patients identified through population sampling from 12 cities, including Vancouver. (18) Overall, the prevalence of COPD stage II or higher was 10.1% ± 4.8% (standard error). In Vancouver, the prevalence of COPD was 9.3% in men and 7.3% in women. (18) Similarly, a longitudinal cohort study using Ontario health administrative data showed an age- and sex-standardized prevalence of COPD in Ontario of 9.5% in 2007. (8)
In a study from Ontario, Hill et al (19) examined patient charts to determine over- and underdiagnosis of COPD. The study examined the charts of patients with a smoking history of at least 20 pack-years and spirometric evidence of COPD, and then matched each patient to 3 patients who did not have spirometric evidence of COPD. Of 382 patients examined, 230 patients had no COPD based on both spirometric results and no diagnosis of COPD on their charts. Of the 152 patients with COPD, 58 (38%) were correctly diagnosed (diagnosis of COPD on chart matching positive spirometry result), 49 (32%) were undiagnosed (no diagnosis of COPD on chart but positive spirometry result), and 45 (30%) were overdiagnosed (diagnosis of COPD on chart but negative spirometry result). (19) These results suggest that both over- and underdiagnosis of COPD may be an issue.
Benefits of Screening for COPD
Given the evidence of COPD underdiagnosis, screening/early detection to identify individuals with COPD may improve their results and health system outcomes by providing treatment that affects morbidity and mortality rates.
In 2008, the Agency for Healthcare Research and Quality (AHRQ) published a review of the evidence on screening for COPD using spirometry. (20) The AHRQ analysis examined English-language literature published up to January 2007 that addressed 8 questions. The questions and a brief summary of the main findings are shown in Table 2.
Table 2: Summary of Evidence from AHRQ Review on Screening for COPD Using Spirometry*.
| Question | Studies Identified | Summary Results |
|---|---|---|
| Does screening for COPD with spirometry reduce morbidity and mortality? | 0 RCTs | No evidence identified |
| What is the prevalence of COPD in the general population? Do risk factors reliably discriminate between high-risk and average-risk populations? | Population-based surveys | Prevalence 4.5%–21.1% depending on definition of COPD |
| What are the adverse effects of screening for COPD with spirometry? | 3 small studies performed in pulmonary function laboratories | Physically safe; some false-positive test results occurred in asymptomatic people |
| Do individuals with COPD detected by screening spirometry have improved smoking cessation rates compared with usual smokers? | 8 RCTs, 2 SRs; only 2 RCTs evaluated independent motivational effect of spirometry | Spirometry did not increase smoking cessation rates; further studies may be needed |
| Does pharmacological treatment, oxygen therapy, or pulmonary rehabilitation for COPD reduce morbidity and mortality? | 43 RCTs, 10 MAs | Pharmacological treatments reduced exacerbations in those with symptomatic severe COPD and had a small effect on all-cause mortality |
| Oxygen therapy reduced mortality in patients with resting hypoxemia | ||
| Pulmonary rehabilitation improved some health status measures | ||
| None of the therapies were tested in patients with airflow obstruction who did not recognize or report symptoms | ||
| What are the adverse effects of COPD treatments? | 12 SRs | Common minor adverse effects included dry mouth, urinary retention, tachycardia, oropharyngeal candidiasis, easy bruising |
| Major adverse effects were rare | ||
| Do influenza and pneumococcal immunizations reduce COPD-associated morbidity and mortality? | 2 SRs | Influenza vaccination reduced COPD exacerbations occurring > 4 weeks after vaccination |
| Pneumococcal vaccination had no statistically significant impact on health outcomes | ||
| What are the adverse effects of influenza and pneumococcal immunizations in patients with COPD? | 2 SRs | Local reactions occurred at the injection site |
Abbreviations: AHRQ, Agency for Healthcare Research and Quality; COPD, chronic obstructive pulmonary disease; MA, meta-analysis; RCT, randomized controlled trial; SR, systematic review.
Source: Lin et al, 2008. (18)
Overall, AHRQ concluded that
screening for COPD using spirometry is likely to identify a predominance of patients with mild-to-moderate airflow obstruction who would not experience additional health benefits if labeled as having COPD. A few individuals with severe airflow obstruction (FEV1 < 50% of predicted) might benefit from pharmacologic treatments that reduce exacerbations. Hundreds of patients would need to have screening spirometry to identify 1 person with COPD whose incremental health benefit over clinical diagnosis would likely be limited to avoidance of a first exacerbation. (20)
Based on these findings, in 2008 the United States Preventive Services Task Force recommended against screening adults for COPD using spirometry. (21) The recommendation was classified as a level D recommendation (moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits). (21)
The United States Preventive Services Task Force, however, recognized the need for further research in the following areas:
The efficacy of various treatments for adults with airflow obstruction who do not recognize or report symptoms, for never smokers, and for smokers younger than 40 years of age.
The effectiveness of primary care screening to detect patients with a clinical diagnosis of severe or very severe COPD.
The diagnostic accuracy of spirometry performed in primary care compared with specialty care settings.
The proportion of patients with previously undiagnosed airflow obstruction who present with a first COPD exacerbation without a clinical diagnosis of COPD. (21)
Since 2008, additional evidence may be available in these areas of uncertainty. Furthermore, based on expert opinion, treatment options and particular medications have improved over the past 5 to 10 years and may lead to greater benefits or additional health benefits.
COPD Medications
A crucial component of COPD treatment is medication. Numerous drugs are involved in the treatment of COPD, including long- and short-acting inhaled bronchodilators (anticholinergics and beta2-agonists), inhaled or oral corticosteroids, methylxanthines, prophylactic antibiotics, mucolytics, and respiratory stimulants. In addition, there are many drug combinations, including combinations of short- and long-acting bronchodilators, combinations of classes of bronchodilators, and combinations of bronchodilators and corticosteroids or bronchodilators and methylxanthines.
The Medical Advisory Secretariat evaluates only nondrug health technologies, so a review of drug therapy for COPD was not included in this project.
Surgical Interventions
Lung volume reduction surgery, bullectomy, and lung transplantation are surgical options that exist for end-stage COPD. These surgical options are invasive and may lead to morbidity and mortality, so only patients with very severe COPD that is not controlled with medical treatment are considered candidates. (22)
Lung volume reduction surgery can be used to treat patients with severe emphysema in which diseased and functionless lung tissue is removed to help improve the functioning of the remaining lung. A 2009 Cochrane Collaboration systematic review by Tiong et al (23) evaluated lung volume reduction surgery for diffuse emphysema. The conclusions from this systematic review were that 90-day mortality was significantly higher in those who received lung volume reduction surgery compared with usual care, but for those who survived longer than 90 days, improvements in lung function, quality of life, and exercise capacity were more likely than for those who received usual care. A subgroup analysis suggested that patients with very impaired lung function and poor diffusing capacity and/or homogeneous emphysema were at high risk for surgical mortality. (23)
Bullectomy can be used to treat COPD patients with a substantial air-filled bulla. The giant bulla is removed to help improve the functioning of the surrounding lung tissue that is being compressed by the bulla. (22;24) The published evidence for bullectomy is based on case series with incomplete follow-up and using a variety of surgical methods. Snider’s review of 22 retrospective case series found that bullectomy is most effective in patients with bullae that are larger than one-third of a hemithorax and compress the adjacent lung tissue, and where FEV1 is less than 50% predicted. Postoperative mortality ranged from 0% to 22.6%. (24;25)
Finally, single- or double-lung transplantation is an option. COPD is one of the most common indications for lung transplantation worldwide. (24;26) Long-term survival data from the Registry of the International Society for Heart & Lung Transplantation found 80% survival at 1 year, 50% at 5 years, and 35% at 10 years. (27)
Methods
This section describes the methods used to scope the mega-analysis; to conduct the systematic reviews of the clinical literature, the economic analysis, and the systematic review of the qualitative literature; and to contextualize the evidence.
A. Mega-Analysis
Project Scope
An initial scoping phase was undertaken to identify any technologies relevant to COPD that impact patient and/or health system outcomes. The scoping process involved identifying and reviewing health technology assessments and systematic reviews of COPD treatment through keyword searches on PubMed and several health technology assessment and systematic review websites (the Wiley Cochrane Library, the Centre for Reviews and Dissemination/International Agency for Health Technology Assessment, and the National Institute for Health and Clinical Excellence). In addition, preliminary searches were conducted in OVID MEDLINE and OVID EMBASE (see Appendix 1 for the search strategies). A number of topics related to COPD were identified during the literature search:
drug therapy for stable COPD and acute exacerbations
hospital-at-home programs for acute exacerbations (early discharge and admission avoidance programs)
invasive ventilation
long-term oxygen therapy
mucous clearing techniques (including mucolytics, chest physiotherapy, and intrapulmonary percussive ventilation)
multidisciplinary care
noninvasive ventilation for acute and chronic respiratory failure
nutritional supplementation
palliative care
pulmonary rehabilitation (for stable COPD and following acute exacerbations)
pulmonary rehabilitation maintenance programs
smoking cessation
surgery (lung volume reduction surgery, bullectomy, and lung transplantation)
telemedicine
vaccinations
Ontario experts in COPD and the members of the Ontario Health Technology Advisory Committee (OHTAC) provided input into the project scope and which topics to include in the analysis.
Disaggregation of Technologies
After the scope of the project was determined, each topic was systematically reviewed in the published literature. Common patient/clinical and health system outcomes of interest were determined a priori so that, where possible, common outcomes were available to compare across technologies. The following outcomes were examined:
complications
dyspnea
emergency department visits
exercise capacity
health-related quality of life
hospital admissions or readmissions
hospital length of stay
lung function
mortality
physician or clinic visits
To accompany the systematic review of the literature, a decision-analytic economic model was developed (methods detailed below). Systematic reviews that yielded high, moderate, or low quality evidence on lung function (measured using FEV1), mortality, and/or hospital admissions were used to populate the economic model. Technologies with outcomes unsuited to the decision-analytic economic model or that had very low quality evidence were not included in the economic model.
Reaggregation
Evidence of effectiveness was combined with evidence of cost-effectiveness, economic feasibility, and information on societal and ethical values obtained from the qualitative literature (methods detailed below) for each of the technologies.
B. Systematic Reviews of Clinical Effectiveness and Safety
For each of the systematic reviews, a literature search was performed using OVID MEDLINE, OVID MEDLINE In-Process and Other Non-Indexed Citations, OVID EMBASE, the Wiley Cochrane Library, EBSCO Cumulative Index to Nursing and Allied Health Literature (CINAHL), and the Centre for Reviews and Dissemination database to identify potential studies. The publication search dates varied by review but typically ranged over 5 to 10 years of literature (specific details are available in the individual reports). Abstracts were reviewed by a single reviewer and, for those studies meeting the eligibility criteria, full-text articles were obtained. Reference lists were also examined for any additional relevant studies not identified through the systematic search.
The inclusion and exclusion criteria listed below were used for all analyses. Some analyses utilized additional criteria specific to the topic of interest, which are detailed in the individual reports.
Research Methods
Inclusion Criteria
English-language full-text reports
HTAs, systematic reviews, meta-analyses, randomized controlled trials (RCTs), and observational studies2
studies performed exclusively using patients with a diagnosis of COPD or studies performed using patients with a mix of conditions if results were reported for COPD patients separately
patients with stable COPD and/or acute exacerbations of COPD as appropriate for the technology
Exclusion Criteria
< 18 years of age
animal studies
duplicate publications
grey literature
Statistical Methods
When possible, results were pooled using Review Manager Version 5.1. (28) Continuous data were pooled to calculate relative risks (RR) using the Mantel-Haenszel test and a random effects model. Dichotomous data were pooled to calculate weighted mean differences using the inverse variance method and a random effects model. When data could not be pooled, the results were summarized descriptively. Analyses using data from RCTs were conducted using intention-to-treat protocols. P values less than 0.05 were considered statistically significant. When possible, clinical significance was defined when the point estimate was greater than or equal to the minimal clinically important difference (MCID). A priori subgroup analyses were planned for many of the analyses based on appropriate differences for each technology. A full description of the method used for each review is available in each individual report.
Quality of Evidence
The quality of each included study was assessed taking into consideration the following study design characteristics:
adequate allocation concealment
randomization (study must include a description of the randomization procedure used and must be a proper method)
power/sample size (adequate sample size based on a priori calculations and underpowered studies were identified, when possible, using post hoc sample size power calculations)
blinding (if double blinding is not possible, a single blind study with unbiased assessment of outcomes was considered adequate for this criterion)
< 20% withdrawals/dropouts
intention-to-treat analysis conducted and done properly (withdrawals/dropouts considered in analysis)
other criteria as appropriate for the particular research question and study design
The quality of the body of evidence was assessed as high, moderate, low, or very low according to the GRADE Working Group criteria (29) as presented below:
Quality refers to criteria such as the adequacy of allocation concealment, blinding, and follow-up.
Consistency refers to the similarity of estimates of effect across studies. If there are important and unexplained inconsistencies in the results, our confidence in the estimate of effect for that outcome decreases. Differences in the direction of effect, the magnitude of the difference in effect, and the significance of the differences guide the decision about whether important inconsistency exists.
Directness refers to the extent to which the interventions and outcome measures are similar to those of interest.
As stated by the GRADE Working Group (29), the following definitions of quality were used in grading the quality of the evidence:
| High | Further research is very unlikely to change confidence in the estimate of effect. |
| Moderate | Further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate. |
| Low | Further research is very likely to have an important impact on confidence in the estimate of effect and is likely to change the estimate. |
| Very Low | Any estimate of effect is very uncertain. |
C. Economic Evaluation
The aim of this evaluation was to assess the cost-effectiveness and impact on the health system of the reviewed COPD treatment strategies.
Cost-Effectiveness Analysis
A cost-utility analysis was conducted using a Markov model. Starting cohorts reflected the various patient populations from the trials of the strategies analyzed. Using clinical parameters and summary estimates of relative risks of (re)hospitalization, mortality, and abstinence from the Medical Advisory Secretariat systematic reviews, incremental cost-effectiveness ratios (ICERs)—that is, costs per quality-adjusted life-year (QALY)—were estimated for each strategy.
Only those technologies for which the systematic reviews yielded hospitalization or mortality data could be included in the model. Furthermore, only those technologies that had high, moderate, or low quality evidence (based on the GRADE criteria) were included. Technologies with very low quality evidence and low quality evidence with nonsignificant results were not included in the model; the estimates of effect were too uncertain to provide useful results. Finally, technologies that were not effective based on the clinical evidence were also not included in the economic model. Given these criteria, the following treatment strategies were included in the model:
smoking cessation programs (intensive counselling, NRT, intensive counselling plus NRT, and the antidepressant bupropion) in moderate COPD in an outpatient setting
multidisciplinary care teams in moderate to severe COPD in an outpatient setting
pulmonary rehabilitation following acute exacerbations in moderate to severe COPD (within 1 month of discharge)
LTOT in severe hypoxemia in COPD in an outpatient setting
ventilation (NPPV in acute respiratory failure due to an acute exacerbation in severe COPD in an inpatient setting and NPPV for weaning COPD patients from IMV in an inpatient setting)
Perspective
The cost-effectiveness analysis (CEA) was taken from the perspective of a publicly funded health care system. Costs from this perspective include drugs covered by the provincial formularies, inpatient costs described by Ontario case costing, and physician fees for services covered by provincial fee schedules. Indirect costs, such as productivity losses, were not considered in the analysis; these were assumed to be minimal considering that the patient population in question is mostly over 65 years of age as reflected in the mean ages from the trials investigated. Costs to family members were beyond the scope of this analysis.
Modelling
Because COPD is a progressive disease, a Markov model was used. The structure of the model, including the transitions between health states, is presented in Figure 2. The circles in the diagram represent different health states. The arrows show the possible patient transitions in a given model cycle. The circular arrows represent cycling within a health state until transition to the next state.
Figure 2: COPD Model Structure*.
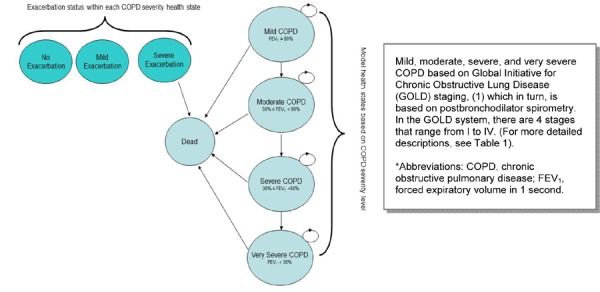
The model comprises different health states based upon the GOLD COPD severity classification. Patients are assigned different costs and utilities depending on the severity of their health during each model cycle. In addition to moving between health states, patients are at risk of acute exacerbations of COPD in each model cycle. During each cycle, patients can have no acute exacerbation, a minor acute exacerbation, or a major exacerbation. For the purpose of the model, a major COPD exacerbation is defined as one requiring hospitalization. Patients are assigned different costs and utilities in each model cycle depending on whether they experience an exacerbation and on the severity of the exacerbation.
Discounting and Time Horizon
An annual discount rate of 5% was applied to costs and QALYs. The time horizon of the model was set to lifetime.
Variability and Uncertainty
Variability and uncertainty in the Markov model were assessed using 1-way and probabilistic sensitivity analyses. Program costs of multidisciplinary care and pulmonary rehabilitation following acute exacerbations were varied in 1-way analyses. Model parameter uncertainty was assessed using probabilistic sensitivity analysis by assigning distributions around the point estimate, and results were presented in the form of probability of cost-effectiveness by ceiling ratio: that is, willingness-to-pay values.
Generalizability
The findings of this economic analysis cannot be generalized to all patients with COPD. The findings may, however, be used to guide decision-making about the specific patient populations addressed in the trials.
Budget Impact Analysis
A budget impact analysis (BIA) was also conducted to project potential incremental costs or potential resources already being incurred in Ontario through the various programs offered in the province. Several assumptions were made to calculate potentially impacted populations for the various strategies investigated. Using provincial data and expert opinion, health system impacts were calculated for each strategy.
Further details of the economic analysis can be found in an associated economic report in this series titled Cost-Effectiveness of Interventions for Chronic Obstructive Pulmonary Disease Using an Ontario Policy Model.
D. Review of Qualitative Literature
Review of Perspectives on Living and Dying with COPD
Literature searches for studies published between January 1, 2000, and November 2010 were performed on November 29, 2010, using OVID MEDLINE; on November 26, 2010, using ISI Web of Science; and on November 28, 2010, using EBSCO Cumulative Index to Nursing and Allied Health Literature (CINAHL). Titles and abstracts were reviewed by a single reviewer and, for those studies meeting the eligibility criteria, full-text articles were obtained. One additional report, highly relevant to the synthesis, appeared in early 2011 during the drafting of this analysis and was included post hoc.
Inclusion Criteria
English-language full-text reports
published from January 1, 2000, through November 2010
primary qualitative empirical research (using any descriptive or interpretive qualitative methodology, including the qualitative component of mixed-methods studies) and secondary syntheses of primary qualitative empirical research
studies addressing any aspect of the experience of living or dying with COPD, from the perspective of persons at risk, patients, health care providers, or informal carers; studies addressing multiple conditions were included if COPD was addressed explicitly
Exclusion Criteria
studies addressing topics other than the experience of living or dying with COPD, from the perspective of persons at risk, patients, health care providers, or informal carers
studies labelled “qualitative” but not using a qualitative descriptive or interpretive methodology (e.g., case studies, experiments, or observational analysis using qualitative categorical variables)
quantitative research (i.e., using statistical hypothesis testing, using primarily quantitative data or analyses, or expressing results in quantitative or statistical terms)
studies that did not pose an empirical research objective or question, or involve the primary or secondary analysis of empirical data
Outcomes of Interest
qualitative descriptions and interpretations (narrative or theoretical) of personal and social experiences of COPD
Further details of the qualitative analysis can be found in an associated report in this series titled Experiences of Living and Dying With COPD: A Systematic Review and Synthesis of the Qualitative Empirical Literature.
E. Contextualization of the Evidence
A COPD Expert Advisory Panel was convened by the Ontario Health Technology Advisory Committee (OHTAC) to assist with contextualizing the results of the systematic reviews and economic analyses. The roles of the panel were as follows:
to provide direction to the Medical Advisory Secretariat on the scope of the project, including relevant background knowledge, grey literature, and relevant subgroup analyses for the evidence review of COPD interventions;
to provide direction on the relevant outcome measures of effectiveness for COPD interventions to help guide the parameters of the systematic review;
to review the systematic evidence-based analyses of the effectiveness of COPD interventions, comment on the accuracy of the interpretation of evidence, and identify any omissions of evidence in the analyses; and
to identify any health system, societal, ethical, or economic issues that were relevant to evaluating the effectiveness of these interventions.
Results of the Evidence-Based Analyses
This section provides a summary of the findings for each of the individual evidence-based reviews. Further details can be found in the individual reports in this COPD series.
1. Influenza and Pneumococcal Vaccinations
Background
Influenza Vaccine
Rates of serious illness due to influenza viruses are high among older people and patients with chronic conditions such as COPD. Complications of influenza infection include viral pneumonia, secondary bacterial pneumonia, and other secondary bacterial infections, such as bronchitis, sinusitis, and otitis media. In viral pneumonia, patients develop acute fever and dyspnea, and may also show signs and symptoms of hypoxia. The incidence of secondary bacterial pneumonia is most common in the elderly and those with underlying conditions such as congestive heart disease and chronic bronchitis.
Healthy people usually recover quickly from influenza. However, influenza is associated with higher risks in the very young or very old and in those with underlying medical conditions such as COPD, heart disease, diabetes, and cancer. It may lead to hospitalization and, in some cases, death. In addition, an influenza infection can exacerbate COPD symptoms or an underlying heart disease.
Every year, the World Health Organization convenes technical meetings in February and September to recommend the selection of virus strains for the seasonal flu vaccine based on the type of influenza viruses that were circulating the previous year.
Pneumococcal Vaccine
The rate of pneumococcal pneumonia in developed countries is still not known due to the lack of accurate diagnostic tests. In the United States Veterans’ Administration Trial, the incidence of pneumococcal pneumonia per 1,000 person years in people aged 55 years and older was 1.7 in those with no underlying disease, 3.4 in those with 1 underlying disease, and 15 in those with 3 underlying diseases.
People with impaired immune systems are susceptible to pneumococcal infection. Young children, elderly people, and patients with underlying medical conditions—including COPD or heart disease, HIV infection, sickle cell disease, and splenectomy—are at higher risk for acquiring pneumococcal pneumonia.
Recommendations for pneumococcal vaccination target most people who are at high risk for invasive pneumococcal disease. However, the use of pneumococcal vaccines in the elderly or high-risk populations is still controversial and has been the subject of many meta-analyses and systematic reviews.
The Centers for Disease Control and Prevention recommends using the 23-valent pneumococcal polysaccharide vaccine in all adults aged 65 years and older and in those adults aged 19 to 64 years of age with underlying medical conditions that put them at greater risk for serious pneumococcal infection and medical conditions, including chronic lung diseases such as COPD, emphysema, and asthma.
Research Questions
What is the effectiveness of the influenza vaccination and the pneumococcal vaccination compared with no vaccination in COPD patients?
What is the safety of these 2 vaccines in COPD patients?
What is the budget impact and cost-effectiveness of these 2 vaccines in COPD patients?
Included Studies
As shown in Figure 3, of the 1,286 citations identified, 2 RCTs met the inclusion/exclusion criteria: 1 for influenza vaccination and 1 for pneumococcal vaccination.
Figure 3: Influenza and Pneumococcal Vaccinations for COPD Citation Flow Chart*.
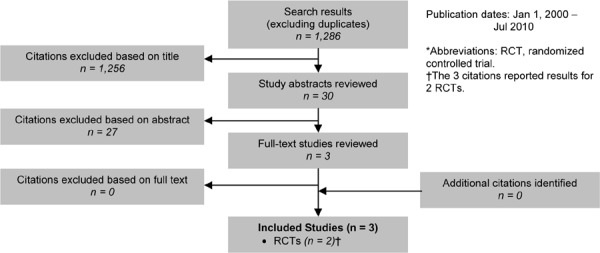
The 2 RCTs3 included a total of 721 participants. The sample size ranged from 125 to 596 people, and the mean age of the patients was between 61 and 76 years. Based on either the GOLD COPD stage criteria or the mean percent predicted FEV1, both studies included people with severe COPD.
The setting for both studies was a single university hospital. In 1 study the control arm received a placebo injection, and in the other study the control arm received no vaccine or a placebo injection.
The individual quality of both studies was high.
Results
Influenza Vaccination
Influenza-Related Acute Respiratory Illness
Influenza vaccination was associated with significantly fewer episodes of influenza-related acute respiratory illness (ARI) (RR, 0.24; 95% confidence interval [CI], 0.09–0.67; P = 0.007).
GRADE: high
When subgrouped by severity of COPD, the incidence density of influenza-related ARIs was significantly reduced in the severe COPD group (RR, 0.1; 95% CI, 0.003–1.1; P = 0.04), but the difference was not significant in the mild and moderate subgroups.
The Kaplan–Meier survival analysis showed a significant difference between the vaccinated and placebo groups in the probability of not acquiring an influenza-related ARI (P = 0.003).
Vaccine Effectiveness
Overall, the vaccine was 76% effective. For the categories of mild, moderate, and severe COPD, effectiveness was 84%, 45%, and 85%, respectively.
GRADE: high
Hospitalizations
A subgroup analysis examined the number of patients with influenza-related ARIs that required hospitalization versus those who were treated in the outpatient setting. This subgroup analysis showed a nonsignificant reduction in hospitalizations due to influenza-related ARIs in the vaccinated group compared with the placebo group (RR, 0.41; 95% CI, 0.08–2.02; P = 0.27).
GRADE: low
Mechanical Ventilation
A subgroup analysis examined the number of patients with influenza-related ARIs that required treatment with mechanical ventilation during hospitalization. This subgroup analysis showed a nonsignificant reduction in the need for mechanical ventilation in the vaccinated group compared with the placebo group (RR, 0.15; 95% CI, 0.01–2.75; P = 0.2).
GRADE: low
Safety/Complications
The most common adverse reactions in the vaccine group were swelling, itching, and pain on contact at the vaccine site, but these symptoms did not require specific treatment and usually lasted less than 48 hours. The incidence of local adverse reactions (27% vs. 6%; P = 0.002), and the incidence of swelling and itching specifically (P = 0.04), were significantly higher in the vaccinated group compared with the placebo group.
Observed systemic reactions were headache, myalgia, fever, and skin rash. There was no significant difference in systemic reactions between the vaccinated group and the placebo group (76% vs. 81%; P = 0.5).
GRADE: low
Economic Model
The influenza vaccination analysis could not be included in the economic model, because the appropriate inputs were not reported in the published literature.
Experiences Concerning Influenza Vaccination (Qualitative Review)
The literature search identified 24,906 citations, of which 218 full-text studies were reviewed. However, none of these studies related to influenza vaccinations.
Pneumococcal Vaccination
Incidence/Episodes of Pneumonia
The Kaplan-Meier survival analysis showed no significant differences in time to first episode of community-acquired pneumonia (CAP) of pneumococcal or unknown etiology between the vaccinated group and the placebo group (log-rank test = 1.15; P = 0.28).
GRADE: high
There were no significant differences in the incidence of global pneumonia (12.7% vs. 12.4%), episodes of global pneumonia (14.4% vs. 15%), or first episodes of CAP (11.1% vs. 11.8%) between the vaccinated group and the placebo group. There was, however, a significant difference in the incidence of pneumococcal pneumonia (0% vs. 1.68%; log-rank test 5.03; P = 0.03).
GRADE: high
Subgroup analyses of age and severity of COPD (based on FEV1) were performed. Significant reductions in episodes of CAP (pneumococcal and unknown etiology combined) were observed for those less than 65 years of age (RR, 0.24; 95% CI, 0.07–0.80; P = 0.02); those with severe COPD (FEV1 < 40%) (RR, 0.52; 95% CI, 0.27–1.01; P = 0.05); and those who fit into both subgroups (i.e., less than 65 years of age and severe COPD) (RR, 0.09; 95% CI, 0.01–0.65; P = 0.02). No significant differences were observed for those who were older than 65 years of age (RR, 1.14; 95% CI, 0.62–2.07; P = 0.67) and those with mild-moderate COPD (FEV1 ≥ 40%) (RR, 1.11; 95% CI, 0.53–2.32; P = 0.78).
Hospitalizations
There was no significant difference in the number of episodes of CAP that required hospitalization between the vaccinated group and the placebo group (76% vs. 81%; P = 0.59).
GRADE: low
Length of Stay
There was no significant difference in the median length of stay (LOS) between the vaccinated group and the placebo group (9.5 days vs. 12 days; P = 0.16).
GRADE: low
Safety
There was no significant difference in the mortality rate between the vaccinated group and the placebo group (about 19% in both groups).
GRADE: low
No patients in either group reported local or systemic reactions to the vaccine.
GRADE: low
Economic Model
The pneumococcal vaccination analysis could not be included in the economic model, because the appropriate inputs were not reported in the published literature.
Experiences Concerning Pneumococcal Vaccination (Qualitative Review)
The qualitative literature search identified 24,906 citations, of which 218 full-text studies were reviewed. However, none of these studies related to pneumococcal vaccinations.
Conclusions
Influenza Vaccine
High quality evidence showed a significant reduction in episodes of influenza-related ARIs in the vaccinated group compared with the placebo group.
Low quality evidence showed nonsignificant reductions in influenza-related ARI hospitalizations and the need for mechanical ventilation in the vaccinated group compared with the placebo group.
Low quality evidence showed a significant increase in local adverse reactions, swelling, and itching in the vaccinated group compared with the placebo group; there was no significant difference, however, in the incidence of systemic reactions between the 2 groups.
Pneumococcal Vaccine
High quality evidence showed a significant decrease in the incidence of pneumococcal pneumonia in the vaccinated group compared with the placebo group; there were no significant differences, however, in the incidence of global pneumonia, episodes of global pneumonia, first episodes of CAP, or time to first episode of CAP between the groups.
Low quality evidence showed no significant differences in hospitalizations due to CAP or hospital LOS between the vaccinated group and the placebo group.
Low quality evidence showed no local or systemic reactions as a result of the vaccine, and the vaccine had no impact on mortality rates.
2. Smoking Cessation
Background
Smoking cessation is the process of discontinuing the practice of inhaling a smoked substance. Smoking cessation programs primarily target tobacco smoking, but may also encompass other substances that can be difficult to stop due to the strong physical addictions or psychological dependencies resulting from their habitual use. Smoking cessation strategies include both pharmacological and nonpharmacological (behavioural or psychosocial) approaches. The basic components of smoking cessation interventions include simple advice, written self-help materials, individual and group behavioural support, telephone quitlines, NRT, and antidepressants. Since addiction to nicotine is a chronic relapsing condition that usually requires several attempts before achieving success, cessation support is usually tailored to individual needs. Nevertheless, it is recognized that, in general, the more intensive the support, the greater the chance of success. In addition, success at quitting smoking decreases with a lack of motivation to quit; the number of pack-years of smoking greater than 10; a lack of social support (e.g., from family and friends); and the presence of psychiatric disorders (such as depression). Smoking cessation can help to slow or halt the progression of COPD.
Research Question
What is the effectiveness and cost-effectiveness of smoking cessation interventions compared with usual care for patients with COPD?
Included Studies
As shown in Figure 4, of the 1,619 citations identified, 13 studies met the inclusion/exclusion criteria: 1 health technology assessment (HTA), 3 systematic reviews, and 9 RCTs.
Figure 4: Smoking Cessation for COPD Citation Flow Chart*.
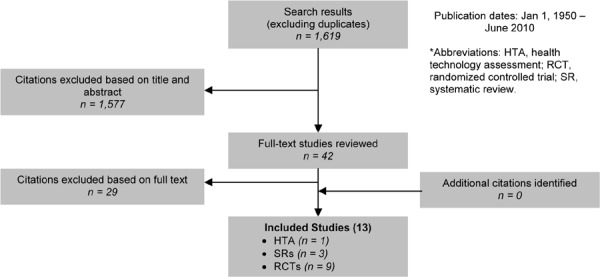
The 9 RCTs included a total of 8,291 participants. The sample size ranged from 74 to 5,887 people, and the mean age of the patients was about 55 years. Based on either the GOLD COPD stage criteria or the mean percent predicted FEV1, 2 studies included people with mild COPD, 3 with mild-to-moderate COPD, 1 with moderate to severe COPD, and 1 with severe to very severe COPD. One study included people at risk of COPD as well as those with mild, moderate, or severe COPD, and the final study did not provide information on the severity of COPD.
Two studies took place in a hospital setting, and the remaining studies in an outpatient setting. Smoking cessation interventions varied across studies and included counselling, pharmacotherapy, or a combination of counselling and pharmacotherapy. The control group received either placebo (for the drug-only trials) or usual care, which was defined as no counselling, pharmacotherapy, or any other type of smoking intervention offered as part of the trial. Since the smoking cessation interventions were very heterogeneous, studies were grouped into categories of similar interventions and pooled if appropriate.
The individual quality of studies was high.
Results
Counselling Versus Usual Care
Two studies that compared counselling and usual care reported abstinence rates. The pooled results showed a statistically significant increase in abstinence in the counselling group compared with the usual care group (RR, 5.85; 95% CI, 3.81–8.97; P = 0.002).
GRADE: moderate
When subgrouped by intensity of counselling, there was a statistically significant increase in abstinence in the intensive counselling (≥ 90 minutes) group compared with the usual care group (RR, 7.70; 95% CI, 4.64–12.79; P < 0.001); however, the increase was nonsignificant between the minimal counselling (< 90 minutes) group and the usual care group (RR, 1.56; 95% CI, 0.65–3.72; P = 0.32). Note that the minimal counselling study was performed in an inpatient setting.
Counselling Plus NRT Versus Usual Care
Three studies that compared counselling plus NRT and usual care reported abstinence rates. The pooled results showed a statistically significant increase in abstinence in the counselling plus NRT group compared with the usual care group (RR, 4.28; 95% CI, 3.51–5.20; P < 0.001).
GRADE: moderate
When subgrouped by intensity of counselling, there was a statistically significant increase in abstinence rates in the intensive counselling (≥ 90 minutes) plus NRT group compared with the usual care group (RR, 4.41; 95% CI, 3.60–5.39; P < 0.001); however, the increase was nonsignificant between the minimal counselling (< 90 minutes) plus NRT group and the usual care group (RR, 2.11; 95% CI, 0.90–4.91; P = 0.08).
Minimal Counselling Plus Antidepressant Versus Usual Care
One study that compared minimal counselling (< 90 minutes) plus antidepressant and usual care reported abstinence rates. The results showed a nonsignificant increase in abstinence rates in the minimal counselling plus antidepressant group compared with the usual care group (RR, 1.91; 95% CI, 0.65–5.61; P = 0.24).
GRADE: low
Minimal Counselling Plus NRT Plus Antidepressant Versus Usual Care
One study that compared minimal counselling (< 90 minutes) plus NRT plus antidepressant and usual care reported abstinence rates. The results showed a nonsignificant increase in abstinence rates in the minimal counselling plus NRT plus antidepressant group compared with the usual care group (RR, 2.25; 95% CI, 0.87–5.85; P = 0.10).
GRADE: low
NRT Versus Placebo
One study that compared NRT and placebo reported abstinence rates. The results showed a statistically significant increase in abstinence in the NRT group compared with the placebo group (RR, 3.01; 95% CI, 1.02–8.89; P = 0.05).
GRADE: moderate
Antidepressant Versus Placebo
Two studies that compared antidepressant and placebo reported abstinence rates. The pooled results showed a statistically significant increase in abstinence in the antidepressant group compared with the placebo group (RR, 2.09; 95% CI, 1.35–3.24; P < 0.001).
GRADE: moderate
When subgrouped by type of antidepressant, there was a statistically significant increase in abstinence rates in the bupropion group compared with the placebo group (RR, 2.01; 95% CI, 1.24–3.24; P = 0.004); however, the increase was nonsignificant between the nortriptyline group and the placebo group (RR, 2.54; 95% CI, 0.87–7.44; P = 0.09).
Economic Model
Comparators and Effect Estimates
The following summary estimates from the systematic review comparing smoking cessation programs versus either usual care or placebo were used in the model to predict long-term outcomes:
-
Cessation rates
– intensive counselling (IC) versus usual care: RR, 7.70 (95% CI, 4.64–12.79; P < 0.001)
– NRT versus placebo: RR, 3.01 (95% CI, 1.02–8.89; P = 0.05)
– IC plus NRT versus usual care: RR, 4.41 (95% CI, 3.60–5.39; P < 0.001)
– bupropion versus placebo: RR, 2.01 (95% CI, 1.24–3.24; P = 0.004)
Mortality and lung function benefits were obtained from the Lung Health Study (30), in which data were analyzed comparing sustained quitters and continuing smokers.
-
Mortality:
– quitters: RR, 0.54
– nonquitters: RR, 1.0
-
Lung function (change in FEV1):
– year 1: quitters, 4.87 mL; nonquitters, –6.81 mL
– year 2 and beyond: quitters, –2.86 mL; nonquitters, –6.19 mL
Resource Use and Costs
Pharmacotherapy was costed based on a typical regimen for smoking cessation as per product monographs in the CPS 2009: Compendium of Pharmaceuticals and Specialties. Counselling was costed based on expert opinion and physician billing in the 2011 Ontario Schedule of Benefits for Physician Services. The cost per program per patient was calculated to be as follows:
usual care, $35.40 (Cdn)
IC, $165.15 (Cdn)
NRT, $203.24 (Cdn)
IC plus NRT, $368.49 (Cdn)
bupropion, $37.92 (Cdn)
CEA Results
All smoking-cessation programs were dominant: that is, they were less expensive and more effective than usual care (usual care was defined as a GP visit).
Using confidence intervals from the systematic review, distributions were assigned to the summary point estimates, and probabilistic sensitivity analyses were run. The probability of smoking cessation programs being cost-effective remained highly probable as the ceiling ratios for willingness to pay increased, since these were dominant strategies.
BIA Results
Ontario pays for intensive counselling through physician billing (Ontario Schedule of Benefits for Physician Services) and for bupropion through the Ontario Drug Benefit formulary. However, NRT is an out-of-pocket expense for smokers. There are 51,029 highly motivated smokers with moderate to severe COPD who could benefit from NRT. Funding NRT could translate to a potential cost to the province of $10 million (Cdn).
Patient Experiences Concerning Smoking Cessation (Qualitative Review)
The qualitative literature search identified 24,906 citations, of which 218 full-text studies were reviewed. Six studies related to COPD patients’ attitudes about and experiences with smoking cessation. Findings suggest that patients’ beliefs about smoking and COPD causation and exacerbation may differ from those of clinicians, and may be difficult to change. COPD patients may feel guilty about how smoking damages their health, and may suffer stigmatization by others—including health care providers—who also perceive the association.
Some patients may prefer nonsmoking explanations, such as genetics, environment, or occupational risks, for their own COPD. Some patients point to inconsistent patterns between smoking and disease in others as evidence that smoking is not necessarily the cause of their own COPD. Patients with COPD sometimes also have inaccurate information or knowledge about the relationship between smoking and COPD, or even about the benefits of smoking cessation. While clinicians might reinforce the negative effects of smoking to improve patient education, smoking cessation advice may backfire if patients feel stigmatized, blamed, or “preached” at. Such interactions may inadvertently drive smokers away from needed health care.
Patients may experience tangible benefits from continuing to smoke. For some, smoking feels like a “friend” that bolsters a sense of well-being and alleviates anxieties. Some patients even feel that smoking alleviates their COPD symptoms.
Some COPD patients feel motivated to quit smoking to improve their health, or for other reasons such as not wanting to burden others or wanting to see their grandchildren grow up. Smoking cessation is difficult, particularly when attempted without professional help. Some patients find smoking cessation unhelpful when it has no perceptible effect on their disease symptoms.
Conclusions
Moderate quality evidence showed a statistically significant increase in abstinence rates in the intensive counselling (≥ 90 minutes) group and the intensive counselling (≥ 90 minutes) and NRT group compared with the usual care group.
Moderate quality evidence showed a statistically significant increase in abstinence rates in the NRT group and the antidepressant bupropion group compared with the placebo group.
Low quality evidence showed no significant differences between the minimal counselling (< 90 minutes) plus antidepressant group and the minimal counselling plus NRT plus antidepressant group compared with the usual care group.
3. Community-Based Multidisciplinary Care
Background
The term multidisciplinary refers to multiple disciplines on a team, and the term interdisciplinary refers to a multidisciplinary team functioning in a coordinated and collaborative manner. The consensus is that a group of multidisciplinary professionals is necessary for optimum specialist management of chronic illness. However, there is little evidence to guide the decision as to which professionals might be needed to optimize the care provided by a multidisciplinary team.
Research Question
What is the effectiveness and cost-effectiveness of multidisciplinary care compared with usual care (single-care provider) for the treatment of stable chronic obstructive pulmonary disease (COPD)?
Included Studies
As shown in Figure 5, of the 2,919 citations identified, 6 RCTs met the inclusion/exclusion criteria.
Figure 5: Multidisciplinary Care for COPD Citation Flow Chart*.
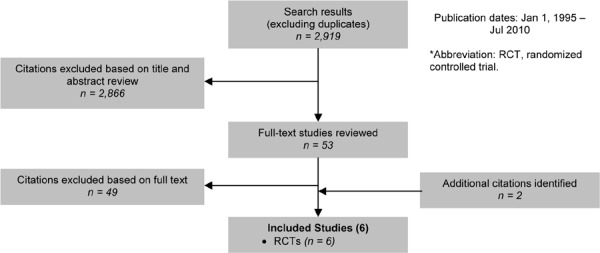
The 6 RCTs included a total of 1,370 participants. The sample size ranged from 40 to 743 people, and the mean age of the patients was between 66 and 71 years. Based on either the GOLD COPD stage criteria or the mean percent predicted FEV1, 3 studies included people with severe COPD and 2 with moderate COPD. The information required to classify the population in the sixth study was not available.
All of the 6 studies were conducted in the community, with 3 completed in the United States. Four studies had multidisciplinary care treatment groups that included a physician. All except 1 reported having a respiratory specialist (i.e., respiratory therapist, specialist nurse, or physician) on the multidisciplinary team. The usual care group comprised a single health care practitioner who may or may not have been a respiratory specialist.
The quality of the studies varied. Common methodological issues included lack of blinding, unclear allocation concealment, greater than 20% loss to follow-up, and unclear use of intention-to-treat analysis.
Results
Hospital Admissions
All-Cause
Four studies reported results of all-cause hospital admissions in terms of number of persons with at least 1 admission during the follow-up period. The pooled results showed a statistically significant 25% reduction in all-cause hospitalizations in the multidisciplinary care group compared with the usual care group (RR, 0.75; 95% CI, 0.64–0.87; P < 0.001).
GRADE: moderate
COPD-Specific
Three studies reported results of COPD-specific hospital admissions in terms of number of persons with at least 1 admission during the follow-up period. The pooled results showed a statistically significant 33% reduction in all-cause hospitalizations in the multidisciplinary care group compared with the usual care group (RR, 0.67; 95% CI, 0.52–0.87; P = 0.002).
GRADE: moderate
Emergency Department Visits
All-Cause
Two studies reported results of all-cause emergency department visits in terms of number of persons with at least 1 visit during the follow-up period. The pooled results showed a statistically nonsignificant reduction in all-cause ED visits in the multidisciplinary care group compared with the usual care group (RR, 0.64; 95% CI, 0.31–1.33; P = 0.24).
GRADE: very low
COPD-Specific
Two studies reported results of COPD-specific ED visits in terms of the number of persons with at least 1 visit during the follow-up period. The pooled results showed a statistically significant 41% reduction in COPD-specific ED visits in the multidisciplinary care group compared with the usual care group (RR, 0.59; 95% CI, 0.43–0.81; P < 0.001).
GRADE: moderate
Mortality
Three studies reported mortality during the study follow-up period (1 year). The pooled results showed a statistically nonsignificant reduction in mortality in the multidisciplinary care group compared with the usual care group (RR, 0.81; 95% CI, 0.52–1.27; P = 0.36).
GRADE: low
Lung Function
Two studies reported results of the percent predicted FEV1 as a measure of lung function. The multidisciplinary care group showed a statistically significant improvement in lung function for up to 1 year compared with the usual group (weighted mean difference [WMD], 3.05; 95% CI, 0.64–5.46; P = 0.01); however, this effect was not maintained at 2 years’ follow-up (WMD, 2.78; 95% CI, –1.82 to 7.37; P = 0.24).
GRADE: very low
Health-Related Quality of Life
Three studies reported HRQOL results based on the St. George’s Respiratory Questionnaire (SGRQ). The studies all showed improvement in the mean change scores (baseline to the end of follow-up) or less deterioration in the multidisciplinary care group compared with the usual care group. The pooled results showed a statistically and clinically4 significant improvement in the total SGRQ score in the multidisciplinary care group compared with the usual care group (WMD, –4.05; 95% CI, –6.47 to –1.63; P = 0.001).
GRADE: low
Economic Model
Comparators and Effect Estimates
The following summary estimate from the systematic review comparing multidisciplinary care and usual care was used in the model to predict long-term outcomes: COPD-specific hospitalizations, (RR, 0.67; 95% CI, 0.52–0.87; P = 0.002).
Resource Use and Costs
Resources reported in the trials investigated were costed and totalled for each trial. The total costs were then averaged to calculate a cost per patient over a program duration range of 6 to 12 months. Resources could include visits with the general physician, dietitian, social worker, physiotherapist, respiratory nurse, or pharmacist. The cost per program per patient was calculated to be $1,041.03 (Cdn).
CEA Results
Assuming a base case cost of $1,041 (Cdn) per multidisciplinary care program per patient, the incremental cost-effectiveness ratio (ICER) was calculated to be $14,123 (Cdn) per QALY. The cost of the program was varied in a 1-way sensitivity analysis to reflect the variation in resource utilization reported in the literature; the ICER increased to $55,322 (Cdn) per QALY.
Using confidence intervals from the systematic review, distributions were assigned to the summary point estimates and probabilistic sensitivity analyses were run. The probability of multidisciplinary care being cost-effective increased as willingness to pay increased.
BIA Results
Family Health Teams (FHTs) often offer chronic disease management programs, including those for COPD. Data from about half the FHTs was reported to the Ministry of Health and Long-Term Care in fiscal year (FY) 2010. The data suggest that 81,289 patients with COPD are accessing COPD management programs within these FHTs, translating to a potential cost to the province of $85 million (Cdn) in FY 2010. However, this estimate does not accurately reflect the current costs to the province because of lack of report by FHTs, lack of capture of programs outside this model of care by any data set in the province, and because the resource utilization and frequency of visits/follow-up phone calls were based on the findings in the literature rather than the actual FHT COPD management programs in place in Ontario. Therefore, MDC resources being utilized in the province are unknown and difficult to measure.
Patient Experiences Concerning Nurse-Led Multidisciplinary Care (Qualitative Review)
The qualitative literature search identified 24,906 citations, of which 218 full-text studies were reviewed. Three studies related to multidisciplinary team care, specifically nurse-led care, for COPD. Two studies, 1 in the Netherlands and 1 in Sweden, explored patient experiences in the context of multidisciplinary—specifically nurse-led—team care for COPD.
The study from the Netherlands reported that patients valued the extra consultation time with nurse practitioners compared with physicians, as well as the time available for education and explaining educational materials. Patients felt safe under the nurse practitioner’s care, but still wanted to maintain their relationship with the specialist physician and be referred smoothly if their care became more complicated. There were mixed views about the appropriate scope of practice for nurse practitioners—some patients favoured a wider scope (e.g., prescribing privileges), while others a narrower scope (e.g., supervision by physicians).
The Swedish study of nurse-led multidisciplinary care for COPD reported that the nurses involved focused their interactions on patients’ medical and physical problems and devoted relatively little time to addressing their psychosocial issues or the prospect of acute exacerbations. Nurses tended to inform patients about self-management and smoking cessation, but tended not to engage in motivational dialogue or articulate an individualized treatment plan with patients.
The third study, from the United Kingdom, found that adopting multidisciplinary care team models for respiratory services involved considerable organizational change. Change could be facilitated by financial incentives (pressures to control costs), teamwork, aligning interests between professionals and administrators, patient involvement, central policy guidance, and adequate support and resources to ensure successful implementation.
Conclusions
Moderate quality evidence showed that multidisciplinary care significantly improved the following health system outcomes compared with usual care: all-cause and COPD-specific hospital admissions, and COPD-specific ED visits.
Low quality evidence showed that multidisciplinary care significantly improved HRQOL compared with usual care.
Very low quality evidence showed that multidisciplinary care significantly improved lung function at 1 year of follow-up, compared with usual care.
Low and very low quality of evidence showed that multidisciplinary care led to a nonsignificant reduction in mortality and all-cause ED visits, compared with usual care.
4. Pulmonary Rehabilitation
Background
Pulmonary rehabilitation refers to a multidisciplinary program of care for patients with chronic respiratory impairment that is individually designed and tailored to optimize physical and social performance and autonomy. Pulmonary rehabilitation is recommended as the standard of care in the treatment and rehabilitation of patients with COPD who remain symptomatic despite treatment with bronchodilators.
Exercise training is the cornerstone of pulmonary rehabilitation programs and may include both aerobic and strength training. Other components of rehabilitation may include psychological support, patient education, nutritional counselling, occupational therapy, medication information, and smoking cessation.
While pulmonary rehabilitation can be delivered in multiple settings for varying durations, questions remain about the optimal site of rehabilitation delivery, components of rehabilitation programs, duration, target populations, and timing of rehabilitation.
For this review, the Medical Advisory Secretariat focused on pulmonary rehabilitation programs defined according to the Cochrane Collaboration definition from the Cochrane review of pulmonary rehabilitation. This defines pulmonary rehabilitation programs as any inpatient, outpatient, or home-based rehabilitation program lasting at least 4 weeks that includes exercise therapy with or without any form of education and/or psychological support delivered to patients with exercise limitation attributable to COPD.
Research Questions
What is the effectiveness and cost-effectiveness of pulmonary rehabilitation compared with usual care for patients with stable COPD?
Does early pulmonary rehabilitation (within 1 month of hospital discharge) in people who had an acute exacerbation of COPD improve outcomes compared with usual care (or no rehabilitation)?
Do maintenance or postrehabilitation programs for patients with COPD who have completed a pulmonary rehabilitation program improve outcomes compared with usual care?
Included Studies
As shown in Figure 6, of the 3,069 citations identified, 29 met the inclusion/exclusion criteria: 1 HTA, 3 systematic reviews, and 25 RCTs.
Figure 6: Pulmonary Rehabilitation for COPD Citation Flow Chart*.
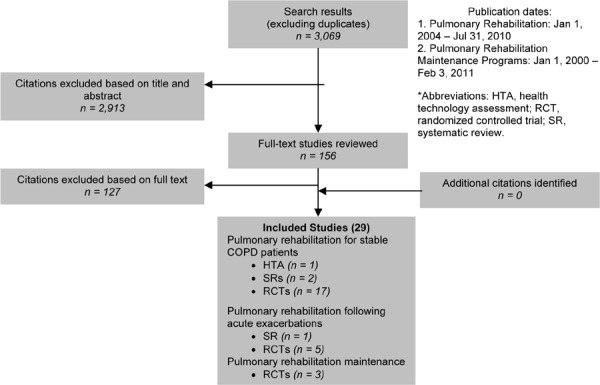
Results
Pulmonary Rehabilitation for Stable COPD
The 17 RCTs included a total of 1,159 participants. The sample size ranged from 28 to 200 people, and the mean age was 66 years. Based on either the GOLD COPD stage criteria or the mean percent predicted FEV1, 13 of the studies included people with severe COPD, 3 with moderate COPD, and 1 with very severe COPD.
Pulmonary rehabilitation programs were delivered in a variety of settings; however, the majority of studies (71%) were conducted in a hospital outpatient setting. All the studies used a usual care control group, and 3 of the 17 studies used a wait-list control group. All of the interventions examined in the studies included a minimum of exercise training. Exercise programs consisted of aerobic training and possibly strength training. Other interventions also included disease education, dietary education/advice, self-care, smoking cessation advice, endurance training, self-management skills, breathing and relaxation exercises, referrals to social services, and psychological support.
The individual quality of the studies varied. Common methodological issues included not conducting analyses using intention-to-treat, lack of blinding, and allocation concealment.
Health-Related Quality of Life
Eight studies reported HRQOL results based on the SGRQ. There was a statistically and clinically5 significant improvement in HRQOL for the pulmonary rehabilitation group compared with the usual care group based on the Total and Activity scores of the SGRQ.
GRADE: moderate
Eight studies reported HRQOL results based on the Chronic Respiratory Questionnaire (CRQ). There was a statistically and clinically6 significant improvement in HRQOL for the pulmonary rehabilitation group compared with the usual care group based on all domains of the CRQ.
GRADE: moderate
Exercise Capacity
Fifteen studies reported results of functional exercise capacity assessment based on the 6 Minute Walking Test (6MWT). The pooled results showed a statistically and clinically7 significant improvement in functional exercise capacity for the pulmonary rehabilitation group compared with the usual care group (WMD, 54.83 m; 95% CI, 35.63–74.03 m; P < 0.001).
GRADE: moderate
Economic Model
This analysis could not be included in the economic model, because the appropriate inputs were not reported in the published literature.
Pulmonary Rehabilitation Following Acute Exacerbations of COPD
A total of 276 participants were included in 5 RCTs. The sample size of the studies ranged from 31 to 97 people, and the mean age of the participants was 68 years. Based on either the GOLD COPD stage criteria or the mean percent predicted FEV1, 3 of the studies included people with severe COPD and 2 included people with moderate COPD.
Pulmonary rehabilitation programs were delivered in a variety of settings. Two studies had outpatient pulmonary rehabilitation programs, 2 studies began with an inpatient program followed by an outpatient program (1 study had a home-based program), and the remaining study had a home-based program for patients who had been admitted to a COPD home from a hospital treatment program. All studies reported a usual care control group. All of the interventions examined in the studies included a minimum of exercise training. Exercise programs consisted of aerobic training, and many also included a strength training component. Other components included in some of the interventions were disease education, dietary education/advice, self-care, smoking cessation advice, endurance training, self-management skills, breathing and relaxation exercises, referrals to social services, and psychological support.
The individual quality of the studies varied. Common methodological issues were unclear randomization and allocation concealment methods, lack of blinding, lack of a priori sample size calculations, and lack of use of intention-to-treat analyses.
Hospital Readmissions
All-Cause
Two studies reported all-cause hospital readmissions. The pooled results showed a nonsignificant reduction in all-cause COPD readmissions in the pulmonary rehabilitation group compared with the usual care group (RR, 0.54; 95% CI, 0.29–1.03; P = 0.06).
GRADE: moderate
COPD-Specific
Three of the studies reported COPD-specific readmissions. The pooled results showed a statistically significant reduction in COPD-specific readmissions in the pulmonary rehabilitation group compared with the usual care group (RR, 0.41; 95% CI, 0.18–0.93; P = 0.03).
GRADE: moderate
Emergency Department Visits
ED visits were reported in 2 studies. The pooled results showed no statistically significant difference between the 2 groups (RR, 0.53; 95% CI, 0.21–1.32; P = 0.17).
Mortality
Mortality was reported in 2 studies. The pooled results showed no statistically significant difference between the 2 groups (RR, 0.60; 95% CI, 0.09–3.88; P = 0.59).
Health-Related Quality of Life
Three studies reported HRQOL results based on the SGRQ. There was a statistically and clinically8 significant improvement in HRQOL, measured by the Total, Impact, and Activity scores, for the pulmonary rehabilitation group compared with the usual care group.
GRADE: moderate
Four studies reported HRQOL results based on the CRQ. There was a statistically and clinically9 significant improvement in HRQOL, measured by all CRQ domains, all for the pulmonary rehabilitation group compared with the usual care group.
GRADE: moderate
Exercise Capacity
Functional exercise capacity measured by the 6MWT was reported in 2 studies. The pooled results showed a statistically and clinically10 significant improvement in exercise capacity in the pulmonary rehabilitation group compared with the usual care group (WMD, 203.14 m; 95% CI, 185.17–221.11 m; P < 0.001).
GRADE: moderate
Economic Model
Comparators and Effect Estimates
The following summary estimate from the systematic review comparing pulmonary rehabilitation with usual care following acute exacerbations of COPD was used in the economic model to predict long-term outcomes in COPD-specific rehospitalization (RR, 0.41; 95% CI, 0.18–0.93; P = 0.03).
Resource Use and Costs
Resources reported in a Toronto-based paper that characterized pulmonary rehabilitation programs in Canada were costed, and the average cost per program per patient was calculated to be $1,527 (Cdn) for patients benefiting from a pulmonary rehabilitation program following an acute exacerbation for a short-term average duration of 4 weeks. Resources varied by province and setting.
CEA Results
Assuming a base case cost of $1,527 (Cdn) per pulmonary rehabilitation program per patient, the ICER was calculated to be $17,938 per QALY. The cost of the program was varied in a 1-way sensitivity analysis to reflect variation in resource utilization reported in the literature. In response, the ICER increased to $56,270 per QALY.
Using confidence intervals from the systematic review, distributions were assigned to the summary point estimates and probabilistic sensitivity analyses were run. The probability of pulmonary rehabilitation being cost-effective increased as the willingness to pay increased.
BIA Results
Data on COPD-specific hospitalization were obtained from Ontario administrative data sets to calculate the potential impact for patients benefiting from pulmonary rehabilitation programs following an exacerbation. There were 22,485 hospitalizations due to COPD in FY 2009. Based on expert opinion, half of hospitalized patients will access pulmonary rehabilitation at least once, which translates to a potential cost of $17 million (Cdn) for the province.
Pulmonary Rehabilitation Maintenance Programs
A total of 284 patients were included in 3 RCTs. The sample size ranged from 48 to 140 people, and the mean age of the participants was about 67 years. Based on either the GOLD COPD stage criteria or the percent predicted FEV1, 2 of the studies included people with moderate COPD, and 1 included people with severe COPD.
All of the maintenance programs were delivered in an outpatient setting. All studies reported using a usual care control group. All of the interventions examined in the studies included a minimum of exercise training. Exercise programs consisted of aerobic training, and 2 of the 3 studies included a strength training component. Two of the studies included unsupervised home exercise as part of the interventions. One of the studies also supplemented the exercise training with weekly educational sessions.
The individual quality of the studies was generally poor. Common methodological issues were unclear randomization and allocation concealment methods, lack of a priori sample size calculations, lack of blinding, and lack of use of intention-to-treat analyses.
Hospitalizations and Length of Stay
Two studies reported hospitalizations and LOS, but the results for these 2 outcomes could not be pooled. Over a 12-month follow-up period, there was no difference in the mean number of hospital admissions per patient or the mean number of days spent in hospital per patient between patients in the maintenance group and the usual care group.
GRADE: low
Exercise Capacity
Two studies reported results of functional exercise capacity assessment based on the 6MWT. The pooled results showed a statistically significant improvement in functional exercise capacity for the maintenance group as compared with the usual care group (WMD, 22.93 m; 95% CI, 5.16–40.71 m; P = 0.01); however, the result was not clinically significant.11 A subgroup analysis that examined the study with a maintenance program of higher intensity showed a marginally clinically11 significant improvement in functional exercise capacity (WMD, 25.88 m; 95% CI, 25.27–26.49 m).
GRADE: low
Health-Related Quality of Life
Two studies reported HRQOL results based on the SGRQ. The results of these studies could not be pooled, as the data were not provided for 1 of the 2 studies. The study that reported results did not find a statistically or clinically12 significant improvement in HRQOL for patients in the maintenance program compared with the usual care group. The authors of the second study noted that there was no significant difference between the groups.
GRADE: low
Economic Model
This analysis could not be included in the economic model because the appropriate inputs were not reported in the published literature.
Experiences Concerning Pulmonary Rehabilitation (Qualitative Review)
The qualitative literature search identified 24,906 citations, of which 218 full-text studies were reviewed. Fourteen studies related to pulmonary rehabilitation for COPD. The major themes identified are summarized here.
Findings from qualitative studies of patients’ attitudes and experiences with pulmonary rehabilitation suggest that pulmonary rehabilitation provides COPD patients with knowledge and techniques to cope with the condition and to control breathing in particular. Better breathing helps patients feel more self-confident and less anxious, and in turn, enables many patients to increase their social participation and activity levels.
Because COPD patients frequently suffer from social isolation, pulmonary rehabilitation provides important opportunities for social interaction. Patients also value enhanced access to health care professionals through their pulmonary rehabilitation programs. Some patients wish for ongoing, rather than time-limited, rehabilitation programs to sustain the benefits and positive experiences.
Obstacles to the pulmonary rehabilitation programs include patients’ low expectations, lack of perceived benefits, and expectation of burdensome exercise. The difficulties of living with COPD, such as exacerbations and overall declining health, can present further barriers to participating in pulmonary rehabilitation.
Conclusions
Stable COPD
Moderate quality evidence showed that pulmonary rehabilitation, including at least 4 weeks of exercise training in persons with COPD, led to clinically and statistically significant improvements in HRQOL as measured by CRQ domains and SGRQ domains compared with usual care.
Moderate quality evidence showed that pulmonary rehabilitation also led to a clinically and statistically significant improvement in functional exercise capacity compared with usual care.
Following Exacerbations
Moderate quality evidence showed that pulmonary rehabilitation after acute exacerbation (within 1 month of hospital discharge) significantly reduced hospital readmissions and led to statistically and clinically significant improvements in HRQOL compared with usual care.
Maintenance Programs
Low quality evidence showed no significant differences between pulmonary rehabilitation maintenance programs and usual care for HRQOL, hospital admissions, and LOS in the hospital.
Low quality evidence showed a statistically but not clinically significant effect of pulmonary maintenance programs on exercise capacity. A subgroup examining the higher quality study with a more intense maintenance program showed a statistically and marginally clinically significant improvement in exercise capacity in the pulmonary maintenance programs group compared with the usual care group.
5. Long-Term Oxygen Therapy
Background
Patients with severe or very severe COPD may experience hypoxemia (low blood oxygen levels). Severe hypoxemia is defined as a PaO2 less than or equal to 55 mm Hg. Moderate hypoxemia is defined by a PaO2 between 56 mm Hg and 65 mm Hg. For the purposes of this report, a mild-to-moderate hypoxemia group was created and refers to PaO2 levels between 56 mm Hg and 74 mm Hg. In patients with hypoxemia, the ventilatory drive is increased to maintain adequate oxygen delivery to tissues. The short-term effects of hypoxemia include greater difficulty breathing, peripheral vascular dilatation with an increase in heart rate and cardiac output, regional pulmonary vasoconstriction, high levels of erythropoietin, and increased hematological viscosity. Prolonged hypoxemia may lead to tissue hypoxia and permanent damage as a result of the adverse effects on organ function and structure.
Oxygen is a treatment option for COPD patients with hypoxemia because these individuals may have difficulty obtaining sufficient oxygen from the air. The provision of oxygen corrects its deficiency in arterial blood and prevents tissue hypoxia.
There are different oxygen sources, including oxygen concentrators, liquid oxygen systems, and oxygen cylinders, each with portable versions. Oxygen is inhaled through a small nasal device or a mask that covers the mouth and nose. Individual needs determine the flow rate, duration of use, method of administration, and oxygen source.
Long-term oxygen therapy, the focus of this analysis, is an extended use of oxygen. Based on Canadian Thoracic Society Guidelines, LTOT of 15 hours per day or more to achieve an oxygen saturation of 90% or more is recommended for patients with stable COPD and severe hypoxemia (PaO2 ≤ 55 mm Hg), or less severe hypoxemia (55 mm Hg < PaO2 ≤ 60 mm Hg) with either bilateral ankle edema, cor pulmonale (right ventricular failure), or hematocrit greater than 56%. In Ontario, oxygen therapy is administered through the Ministry of Health and Long-Term Care’s Assistive Devices Program. The eligibility criteria for LTOT in Ontario are consistent with Canadian Thoracic Society guidelines, but also include patients with persistent hypoxemia (PaO2, 56–60 mm Hg) and exercise-limited hypoxemia documented to improve with supplemental oxygen, or nocturnal hypoxemia, as well as patients with exertional hypoxemia without hypoxemia at rest.
There has been limited work on the safety of oxygen therapy. Use of LTOT in the presence of a fire source, such as a lit cigarette, can accelerate a fire that may lead to facial burns. Other safety hazards include falls related to oxygen tubing and underusing oxygen. As well, patients with type 2 respiratory failure using high doses of oxygen could further elevate their tissue carbon dioxide levels.
Research Question
What is the effectiveness, cost-effectiveness, and safety of LTOT compared with no LTOT in patients with COPD, when stratified by severity of hypoxemia?
Included Studies
In addition to the standard inclusion criteria detailed in the methods section, studies that included COPD patients with hypoxemia were included in this section of the review. As shown in Figure 7, of the 1,096 citations identified, 8 met the inclusion/exclusion criteria: 3 systematic reviews, 3 RCTs, and 2 observational studies.
Figure 7: Long-Term Oxygen Therapy for COPD Citation Flow Chart*.
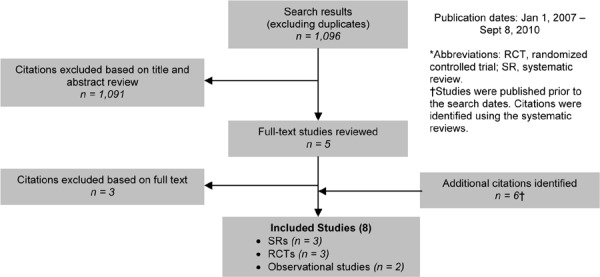
Since the RCT evidence did not provide results on health system outcomes, observational studies reporting on health system outcomes were included. In addition to the systematic search described above, a nonsystematic search using MEDLINE was conducted to identify additional citations that examined the impact of LTOT on HRQOL. From this search, an additional 3 prospective observational studies were identified.
The 8 studies included a total of 802 participants. The sample size ranged from 19 to 312 people, and the mean age of the patients was about 64.5 years. Based on either the GOLD COPD stage criteria or the mean percent predicted FEV1, 3 studies included people with severe COPD and 5 with very severe COPD. The analysis was divided into 2 categories based on the severity of hypoxemia. Studies were classified as either mild-to-moderate hypoxemia (55 mm Hg < PaO2 ≤ 74 mm Hg) or severe hypoxemia (PaO2 ≤ 55 mm Hg). One RCT included patients with mild hypoxemia, 1 with moderate hypoxemia, and 1 with severe hypoxemia. The observational studies, which reported health system outcomes, included patients with severe hypoxemia in the LTOT group and patients with mild hypoxemia in the no LTOT group. Finally, the data on HRQOL from the prospective observational studies included patients with severe hypoxemia.
All 8 studies were conducted in the community with stable COPD patients. Patients in the LTOT group received oxygen therapy for about 15 hours per day (this time may include day and night). The no-LTOT group received usual care.
The individual quality of the studies varied between studies. This analysis included both RCTs and observational studies. Common methodological issues in the RCT evidence were lack of allocation concealment, lack of information on randomization methods, and sparse data. Common limitations in the observational evidence were heterogeneity in the comparison groups and sparse data.
Results
Severe Hypoxemia (PaO2 ≤ 55 mm Hg)
Mortality
One RCT reported mortality. The study showed a borderline significant reduction in mortality in the LTOT group compared with the no-LTOT group (RR, 0.68; 95% CI, 0.46–1.00; P = 0.05).
GRADE: low
Lung Function
One RCT reported lung function measured by FEV1. The study showed that among survivors, patients on LTOT showed an improvement in FEV1 compared with no-LTOT therapy (WMD, 0.08 L; 95% CI, 0.04–0.12 L; P < 0.001).
GRADE: very low
Health-Related Quality of Life
Three studies reported HRQOL based on the SGRQ and CRQ. The results could not be pooled. The following results are based on before-after comparisons of individuals who were put on LTOT (the original trials were RCTs, but only the LTOT arm was examined for this outcome). One study that reported the SGRQ showed a clinically significantly improvement in the mean change in the SGRQ total score at 2 weeks and 3 months. There were clinically significant improvements in the Impacts domain (2 weeks), Activities and Impacts domains (3 months), and Symptoms and Impacts domains (6 months). Statistical significance was not reported by this study.
Two additional studies reported HRQOL using the CRQ. The first study showed clinically and statistically significant improvements in some CRQ domains for males and females in the short and long term. The second showed statistically significant improvements in CRQ domains and total scores at 2 and 6 months (clinical significance was not defined).
GRADE: low to very low
Hospitalizations
One observational study reported hospitalizations. The study showed an increase in hospitalizations in the LTOT group compared with the no-LTOT group (percentage of patients free of hospitalizations, 38% vs. 77%; P = 0.01). In this study, patients in the no-LTOT group had mild-to-moderate hypoxemia, while patients in the LTOT group had severe hypoxemia.
GRADE: very low
Length of Stay
One RCT reported hospital LOS. The study showed no difference between the LTOT and no-LTOT groups (no data reported).
GRADE: low
Economic Model
Comparators and Effect Estimates
The following summary estimate from the systematic review comparing LTOT and no LTOT was used in the model to predict long-term outcomes: mortality (RR, 0.68; 95% CI, 0.46–1.0; P = 0.05).
Resource Use and Costs
The Ministry of Health and Long-Term Care Assistive Devices Program supplies LTOT resources and equipment to patients with severe hypoxemia. According to the Assistive Devices Program, the average cost per patient was $2,261 (Cdn) in FY 2006. Resources offered through the program include home assessment, 24-hour emergency service, maintenance and repair, training and education, oxygen supply system, and disposables (i.e., nasal cannula and tubing). This average cost of LTOT to patients with severe hypoxemia was assumed to be an annual incurrence in the model, since patients would be expected to remain indefinitely on LTOT.
CEA Results
Assuming a base case cost of $2,261 (Cdn) per year per patient, the ICER was calculated to be $38,993 (Cdn) per QALY. Using confidence intervals from the systematic reviews, distributions were assigned to the summary point estimates, and probabilistic sensitivity analyses were run. The probability of LTOT being cost-effective increased as the willingness to pay increased.
BIA Results
Data from the Assistive Devices Program suggested that 28,654 patients with severe hypoxemia accessed LTOT in FY 2006, which translates to a cost of $65 million (Cdn) for the province.
Mild-to-Moderate Hypoxemia (55 mm Hg < PaO2 ≤ 74 mm Hg)
Mortality
Two RCTs reported mortality. The results were not pooled due to differences in the length of the intervention/follow-up (3 years vs. 7 years). At 3 years, there was a nonsignificant increase in mortality between the LTOT and no-LTOT groups (RR, 1.33; 95% CI, 0.36–4.90; P = 0.66). At 7 years, there was a nonsignificant increase in mortality in the LTOT group compared with the no-LTOT group (RR, 1.17; 95% CI, 0.84–1.62; P = 0.35).
GRADE: low (3 and 7 years)
Lung Function
Two RCTs reported lung function measured by percent predicted FEV1 among survivors. The results were not pooled due to differences in the length of intervention/follow-up (1 year vs. 7 years). At 1 year, there was a nonsignificant improvement in lung function in the LTOT group compared with the no-LTOT group (WMD, –3.50%; 95% CI, –11.06 to 4.06%; P = 0.36). At 7 years, there was a nonsignificant improvement in lung function in the LTOT group compared with the no-LTOT group (WMD, –1.70%; 95% CI, –6.59 to 3.19%; P = 0.50).
GRADE: very low
Exercise Capacity
One RCT reported functional exercise capacity measured by endurance time (minutes). The results showed a nonsignificant improvement in the LTOT group compared with the no-LTOT group (WMD, –1.9 minutes; 95% CI, –4.52 to 0.72 minutes; P = 0.16).
GRADE: very low
Dyspnea
One RCT reported dyspnea measured using the Borg Scale. The study showed a nonsignificant improvement in the LTOT group compared with the no-LTOT group (WMD, –1.20; 95% CI, –2.51 to 0.11; P = 0.07).
GRADE: very low
Economic Model
Due to the low/very low quality of evidence and nonsignificant results, LTOT for mild-to-moderate hypoxemia was not included in the economic model.
Experiences Concerning Oxygen Therapy (Qualitative Review)
The qualitative literature search identified 24,906 citations, of which 218 full-text studies were reviewed. Three studies related to COPD patients’, informal caregivers’, and health care providers’ experiences with oxygen therapy for COPD; 2 relevant themes were identified.
The first theme related to lay beliefs about oxygen therapy. Findings showed that patients gained a sense of independence with use, symptom mastery, improved sleep, and a source of reassurance from the presence of oxygen therapy.
The second theme related to adherence and covered numerous areas, including functional limitation, health benefits, symptom relief, social pressure, and self-management. Increased adherence was associated with:
modifying the functional limitations of the heavy oxygen equipment;
the perception of improved health benefits;
worsening of symptoms;
symptom relief; and
social pressures for use (e.g., family, friends, or physician).
Decreased adherence was associated with avoiding the drawbacks of use (e.g., activity modification) and embarrassment. Patients display a series of decision-making steps as they come to terms with their use of oxygen therapy.
Conclusions
Severe Hypoxemia (PaO2 ≤ 55 mm Hg)
Low quality evidence showed a borderline significant reduction in mortality in the LTOT group compared with the no-LTOT group.
Very low quality evidence showed a significant improvement in FEV1 in the LTOT group compared with the no-LTOT group.
Low to very low quality evidence showed a significant improvement in HRQOL as measured by some domains of the SGRQ and the CRQ in the LTOT group compared with the no-LTOT group.
Low quality evidence showed an increase in hospitalizations in the LTOT group compared with the no-LTOT group, but there was no difference in hospital LOS between the 2 groups.
Mild-to-Moderate Hypoxemia (55 mm Hg < PaO2 ≤ 74 mm Hg)
Low quality evidence showed no difference in mortality in the LTOT group compared with the no-LTOT group at 3 and 7 years of follow-up.
Very low quality evidence showed nonsignificant improvements in percent predicted FEV1, endurance time, and dyspnea in the LTOT group compared with the no-LTOT group.
6. Noninvasive Positive Pressure Ventilation for Acute Respiratory Failure
Background
Acute hypercapnic respiratory failure frequently occurs in COPD patients experiencing acute exacerbations of COPD. Hypercapnic respiratory failure occurs due to a decrease in the drive to breathe, typically due to increased work to breathe in COPD patients.
There are several treatment options for acute respiratory failure. Usual medical care (UMC)13 attempts to facilitate adequate oxygenation and treat the cause of the exacerbation. It typically consists of supplemental oxygen, and a variety of medications such as bronchodilators, corticosteroids, and antibiotics. The failure rate of UMC has been estimated to occur in 10% to 50% of cases.
The alternative to UMC is mechanical ventilation, either IMV or noninvasive ventilation. IMV involves sedating the patient, creating an artificial airway through endotracheal intubation, and attaching the patient to a ventilator. While this approach provides airway protection and direct access to drain sputum, it can lead to substantial morbidity and risks, including tracheal injuries and VAP.
While noninvasive ventilation can be done by either positive or negative pressure, noninvasive negative pressure ventilation (such as the iron lung) is no longer in use in Ontario. NPPV provides ventilatory support through a facial or nasal mask and reduces inspiratory work. NPPV can often be used intermittently for short periods of time to treat respiratory failure, allowing patients to continue to eat, drink, talk, and participate in their own treatment decisions. In addition, patients do not require sedation, airway defence mechanisms and swallowing functions are maintained, trauma to the trachea and larynx are avoided, and the risk for VAP is reduced. Common complications with NPPV are damage to facial and nasal skin, higher incidence of gastric distension with aspiration risk, sleeping disorders, and conjunctivitis. In addition, NPPV does not allow direct access to the airway to drain secretions, requires patients to cooperate, and (due to potential discomfort) compliance and tolerance may be low.
In addition to treating acute respiratory failure, NPPV can be used to wean patients from IMV through the gradual removal of ventilation support until the patient can breathe spontaneously. Five percent to 30% of patients have difficulty weaning. Tapering levels of ventilatory support to wean patients from IMV can be achieved using either IMV or NPPV. Use of NPPV helps to reduce the risk of VAP by shortening the amount of time the patient is intubated.
Following extubation from IMV, acute respiratory failure may recur, leading to extubation failure and the need for reintubation, which has been associated with increased risk of nosocomial pneumonia and mortality. To avoid these complications, NPPV has been proposed to help prevent acute respiratory failure from recurring and/or to treat respiratory failure when it recurs, thereby preventing the need for reintubation.
Research Questions
-
What is the effectiveness, cost-effectiveness, and safety of NPPV for the treatment of acute hypercapnic respiratory failure due to acute exacerbations of COPD compared with:
– usual medical care, and
– IMV?
-
What is the effectiveness, cost-effectiveness, and safety of NPPV compared with IMV in COPD patients after IMV for the following purposes:
– weaning COPD patients from IMV,
– preventing acute respiratory failure in COPD patients after extubation from IMV, and
– treating acute respiratory failure in COPD patients after extubation from IMV?
Included Studies
As shown in Figure 8, of the 2,585 citations identified, 31 met the inclusion/exclusion criteria: 14 systematic reviews and 17 RCTs.
Figure 8: NPPV for Acute Respiratory Failure Citation Flow Chart*.
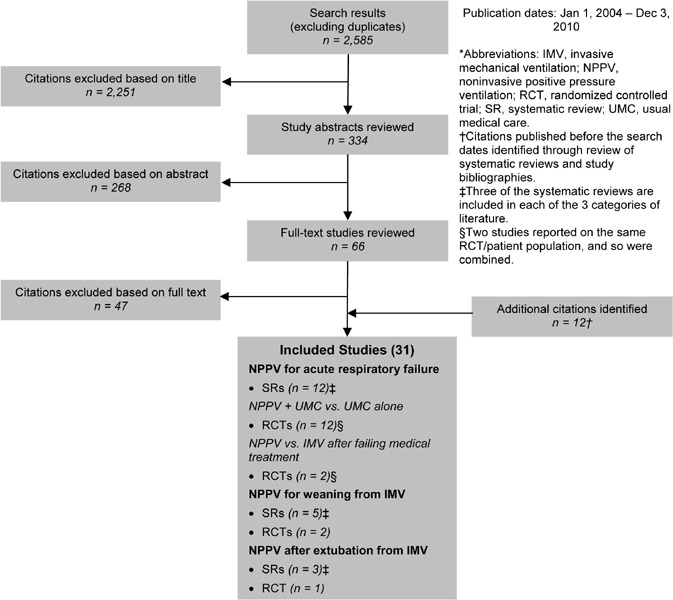
Results
NPPV for Acute Respiratory Failure Due to Acute Exacerbations of COPD
Thirteen14 RCTs evaluating the effectiveness of NPPV for the treatment of acute respiratory failure due to acute exacerbations of COPD were identified. The following comparisons were examined:
NPPV plus UMC versus UMC alone (11 RCTs)
NPPV versus IMV (2 RCTs)
NPPV plus UMC Versus UMC for First-Line Treatment
The 11 RCTs included a total of 1,000 participants. The sample size ranged from 23 to 342 people, and the mean age of the patients ranged from 60 to 72 years. Based on either the GOLD COPD stage criteria or the mean percent predicted FEV1, 4 of the studies included people with severe COPD; inadequate information was available to classify the remaining 7 studies by COPD severity. The severity of the respiratory failure was classified into 4 categories using the study population mean pH level as follows: mild (pH ≥ 7.35), moderate (7.30 ≤ pH < 7.35), severe (7.25 ≤ pH < 7.30), and very severe (pH < 7.25). Based on these categories, 3 studies included patients with a mild respiratory failure, 3 with moderate respiratory failure, 4 with severe respiratory failure, and 1 with very severe respiratory failure.
The studies were conducted either in the intensive care unit (ICU) (3 of 11 studies) or general or respiratory hospital wards (8 of 11 studies) with patients in the NPPV group receiving bilevel positive airway pressure (BiPAP) ventilatory support, although 1 study used pressure support ventilation and 1 used volume cycled ventilation. Patients received ventilation through nasal, facial, or oronasal masks. All studies specified a protocol or schedule for NPPV delivery, but this varied substantially across the studies. For example, some studies restricted the amount of ventilation per day (e.g., 6 hours per day) and the number of days it was offered (e.g., maximum of 3 days), whereas other studies provided patients with ventilation for as long as they could tolerate it and recommended it for much longer periods of time (e.g., 7–10 days). These differences are an important source of clinical heterogeneity between studies. In addition to NPPV, all patients in the NPPV group also received usual medical care. Usual medical care varied between studies, but common interventions included supplemental oxygen, bronchodilators, corticosteroids, antibiotics, diuretics, and respiratory stimulators.
The individual quality of the studies varied. Common methodological issues included lack of blinding, allocation concealment, and small sample sizes.
Need for Endotracheal Intubation
Eleven studies reported the need for endotracheal intubation. The pooled results showed a significant reduction in the need for endotracheal intubation in the NPPV plus UMC group compared with the UMC group (RR, 0.38; 95% CI, 0.28–0.50; P < 0.001). When subgrouped by severity of respiratory failure, the results remained significant for the mild, severe, and very severe respiratory failure groups.
GRADE: moderate
Inhospital Mortality
Nine studies reported inhospital mortality. The pooled results showed a significant reduction in inhospital mortality in the NPPV plus UMC group compared with the UMC group (RR, 0.53; 95% CI, 0.35–0.81; P = 0.003). When subgrouped by severity of respiratory failure, the results remained significant for the moderate and severe respiratory failure groups.
GRADE: moderate
Hospital Length of Stay
Eleven studies reported hospital LOS. The pooled results showed a significant decrease in mean LOS for the NPPV plus UMC group compared with the UMC group (WMD, –2.68 days; 95% CI, –4.41 to –0.94 days; P = 0.002). When subgrouped by severity of respiratory failure, the results remained significant for the mild, severe, and very severe respiratory failure groups.
GRADE: moderate
Complications
Five studies reported complications. Common complications in the NPPV plus UMC group included pneumonia, gastrointestinal disorders or bleeds, skin abrasions, eye irritations, gastric insufflations, and sepsis. Similar complications were observed in the UMC group, including pneumonia, sepsis, gastrointestinal disorders or bleeds, pneumothorax, and complicated endotracheal intubations. Many of the more serious complications in both groups occurred in patients who required endotracheal intubation. Three of the studies compared complications in the NPPV plus UMC group and UMC groups. While the data could not be pooled, overall the NPPV plus UMC group experienced fewer complications than the UMC group.
GRADE: low
Tolerance/Compliance
Eight studies reported patient tolerance or compliance with NPPV. NPPV intolerance ranged from 5% to 29%. NPPV tolerance was generally higher for patients with more severe respiratory failure. Compliance with the NPPV protocol was reported by 2 studies, which showed compliance decreases over time, even over short periods, such as 3 days.
Economic Model
Comparators and Effect Estimates
The following summary estimate from the systematic review comparing NPPV plus UMC with UMC was used in the model to predict long-term outcomes: inpatient mortality (RR, 0.53; 95% CI, 0.35–0.81; P = 0.003).
Resource Use and Costs
The Ontario Case Costing Initiative collects cost data for acute inpatient, day surgery, and ambulatory care cases from participating hospitals. This provides a standard data set for hospitalization costs in Ontario. Cost per diem or per average LOS can be obtained by most responsible diagnosis and principal procedure. Codes were identified through Canadian Institute for Health Information reference, and cost per diem for noninvasive ventilation in COPD was obtained. The cost for UMC for a COPD hospitalization was obtained from Canadian literature. The following estimates were used:
NPPV, $864 per diem
UMC, $1,009 per diem
Based on average LOSs reported in the trials, total costs for the hospitalization episode of each arm were calculated.
CEA Results
The NPPV plus UMC strategy was dominant; that is, it was cheaper and more effective, as reflected by the clinical evidence of significant inhospital days avoided.
BIA Results
Based on expert opinion, 15% of the patient population at risk is eligible for ventilation and 50% will choose to be ventilated. These estimates suggest that 11,163 patients can benefit from NPPV, which translates into a cost savings to the province from the hospital perspective of $42 million (Cdn).
NPPV Versus IMV
A total of 205 participants were included in 2 studies. The sample sizes were 49 and 156 people. The mean age of the patients was 71 to 73 years in 1 study, and the median age was 54 to 58 years in the other. Based on either the GOLD COPD stage criteria or the mean percent predicted FEV1, patients in 1 study had very severe COPD. The COPD severity could not be classified in the second study. Both studies included patients with very severe respiratory failure (mean pH < 7.23). One study enrolled patients with acute respiratory failure due to acute exacerbations of COPD who had failed medical therapy. The patient population was not clearly defined in the second study, and it was not clear whether patients had to have failed medical therapy before entry into the study.
Both studies were conducted in the ICU. Patients in the NPPV group received BiPAP ventilatory support through nasal or full facial masks. Patients in the IMV group received pressure support ventilation.
Common methodological issues included small sample size, lack of blinding, and unclear methods of randomization and allocation concealment. Due to uncertainty about whether both studies included the same patient population and substantial differences in the direction and significance of the results, the results of the studies were not pooled.
Mortality
Both studies reported ICU mortality. Neither study showed a significant difference in ICU mortality between the NPPV and IMV groups; 1 study, however, showed a higher mortality rate in the NPPV group (21.7% vs. 11.5%; P value not reported), while the other study showed a lower mortality rate in the NPPV group (5.1% vs. 6.4%; P = 0.93). One study reported 1-year mortality and showed a nonsignificant reduction in mortality in the NPPV group compared with the IMV group (26.1% vs. 46.1%; P = 0.24).
GRADE: low to very low
Intensive Care Unit Length of Stay
Both studies reported LOS. The results were inconsistent. One study showed a statistically significant shorter LOS in the NPPV group compared with the IMV group (mean [standard deviation (SD)] = 5.00 [1.35] days vs. 9.29 [3.00] days; P < 0.001), whereas the other showed a nonsignificantly longer LOS in the NPPV group compared with the IMV group (mean [SD] = 22 [19] days vs. 21 [20] days; P = 0.86).
GRADE: very low
Duration of Mechanical Ventilation
Both studies reported the duration of mechanical ventilation (including both invasive and noninvasive ventilation). The results were inconsistent. One study showed a statistically significant shorter duration of mechanical ventilation in the NPPV group compared with the IMV group (mean [SD] = 3.92 [1.08] days vs. 7.17 [2.22] days; P < 0.001), whereas the other showed a nonsignificantly longer duration of mechanical ventilation in the NPPV group compared with the IMV group (mean [SD] = 16 [19] days vs. 15 [21] days; P = 0.86).
GRADE: very low
Complications
Both studies reported VAP and tracheotomies. Both studies showed a reduction in VAP in the NPPV group compared with the IMV group, but the results were significant in only 1 study (13% vs. 34.6%; P = 0.07 and 6.4% vs. 37.2%; P < 0.001). Similarly, both studies showed a reduction in tracheotomies in the NPPV group compared with the IMV group, but the results were significant in only 1 study (13% vs. 23.1%; P = 0.29; and 6.4% vs. 34.6%; P < 0.001).
GRADE: very low
Other Outcomes
One of the studies followed patients for 12 months, at the end of which patients in the NPPV group had a significantly lower rate of needing de novo oxygen supplementation at home. In addition, the IMV group experienced significant increases in functional limitations due to COPD, while no increase was seen in the NPPV group. Finally, no significant differences were observed for hospital readmissions, ICU readmissions, and patients with an open tracheotomy between the NPPV and IMV groups.
Economic Model
Due to the low/very low quality of evidence and inconsistent results that could not be pooled, these results were not included in the economic model.
NPPV for Weaning COPD Patients from IMV
The 2 RCTs included a total of 80 participants. The sample sizes were 30 and 50 people, and the mean age of the patients ranged from 58 to 69 years. Based on either the GOLD COPD stage criteria or the mean percent predicted FEV1, both studies included patients with very severe COPD. Both studies also included patients with very severe respiratory failure (mean pH < 7.23). COPD patients receiving IMV were enrolled in the study if they failed a spontaneous breathing test (T-piece weaning trial), so they could not be directly extubated from IMV.
Both studies were conducted in the ICU. Patients in the NPPV group were weaned using either BiPAP or pressure support ventilation NPPV through a face mask, while patients in the IMV weaning group received pressure support ventilation. In both cases, weaning was achieved by tapering the ventilation level slowly.
The individual quality of the studies varied. Common methodological problems included unclear randomization methods and allocation concealment, lack of blinding, and small sample size.
Mortality
Both studies reported mortality. The pooled results showed a significant reduction in ICU mortality in the NPPV group compared with the IMV group (RR, 0.47; 95% CI, 0.23–0.97; P = 0.04).
GRADE: moderate
Intensive Care Unit Length of Stay
Both studies reported LOS. The pooled results showed a nonsignificant reduction in ICU LOS in the NPPV group compared with the IMV group (WMD, –5.21 days; 95% CI, –11.60 to 1.18 days; P = 0.11).
GRADE: low
Duration of Mechanical Ventilation
Both studies reported the duration of mechanical ventilation (including both invasive and noninvasive ventilation). The pooled results showed a nonsignificant reduction in duration of mechanical ventilation (WMD, –3.55 days; 95% CI, –8.55 to 1.44 days; P = 0.16).
GRADE: low
Nosocomial Pneumonia
Both studies reported nosocomial pneumonia. The pooled results showed a significant reduction in nosocomial pneumonia in the NPPV group compared with the IMV group (RR, 0.14; 95% CI, 0.03–0.71; P = 0.02).
GRADE: moderate
Weaning Failure
One study reported a significant reduction in weaning failure in the NPPV group compared with the IMV group, but not the data. In this study, 1 of the 25 patients in the NPPV group and 2 of the 25 patients in the IMV group could not be weaned after 60 days in ICU.
GRADE: moderate
Economic Model
Comparators and Effect Estimates
The summary estimate of inpatient mortality (RR, 0.47; 95% CI, 0.23–0.97; P = 0.04) from the systematic review comparing NPPV versus IMV was used in the model to predict long-term outcomes.
Resource Use and Costs
The Ontario Case Costing Initiative collects cost data for acute inpatient, day surgery, and ambulatory care cases from participating hospitals. This provides a standard data set for hospitalization costs. Cost per diem or per average LOS can be obtained by most responsible diagnosis and principal procedure. The following per diem estimates for IMV and NPPV in COPD were used:
IMV, $1,679 per diem
NPPV, $864 per diem
Based on average LOS reported in the trials, total costs for the hospitalization episode of each arm were calculated.
CEA Results
Weaning with NPPV was a dominant strategy: that is, the strategy is cheaper and more effective than weaning with IMV (as reflected by the reduced inpatient mortality in the study group).
BIA Results
Based on expert opinion, 15% of the patient population at risk is eligible for ventilation. Of those, 50% will choose to be ventilated, and 15% will fail spontaneous breathing tests. Therefore, an estimated 1,435 patients can benefit from weaning with NPPV, which translates into a cost savings to the province from the hospital perspective of $12 million (Cdn).
NPPV After Extubation of COPD Patients from IMV
The literature was reviewed to identify studies that examine the effectiveness of NPPV compared with usual medical care in preventing recurrence of acute respiratory failure after extubation from IMV or treating acute respiratory failure that recurred after extubation from IMV. None of the studies identified included COPD patients only or reported results for preventing acute respiratory failure after extubation for COPD patients separately.
Reintubation
One study discussed the treatment of acute respiratory failure that recurred within 48 hours of extubation from IMV in COPD patients. This study included 221 patients, of whom 23 had COPD. A post hoc subgroup analysis examined the rate of reintubation in the COPD patients only. A nonsignificant reduction in the rate of reintubation was observed in the NPPV group compared with the usual medical care group (7 of 14 patients vs. 6 of 9 patients, P = 0.67).
GRADE: low
Economic Model
Due to the low quality of evidence and nonsignificant results, the results on the use of NPPV in COPD patients to treat acute respiratory failure after extubation from IMV were not included in the economic model.
Experiences Concerning Ventilation (Qualitative Review)
The qualitative literature search identified 24,906 citations, of which 218 full-text studies were reviewed. Three studies related to patients’ experiences with noninvasive (2 studies) and invasive (1 study) ventilation.
Findings showed both adverse and beneficial effects in COPD patients for both invasive and noninvasive ventilation. Potential adverse effects include patients feeling trapped by the machine, both literally and figuratively; feeling dependent on it; and feeling shut in or suffocated by the mask. Difficulties moving, communicating, and making choices bring further distress.
In terms of advantages, ventilation provides the much-appreciated benefit of improved breathing and regaining strength with time. Patients develop the ability to cope with the mask and machine, just as they regain strength and willpower, realize their situation, and to some extent redefine themselves. Clinicians’ presence and encouragement are highly valued and improve the ability to cope.
Other findings were that COPD patients who become candidates for ventilation are quite vulnerable, both mentally and physically (breathless, anxious, incapacitated, exhausted); they typically have little knowledge of ventilation technology before it is offered to them.
The study on invasive ventilation found that patients often experience a period of amnesia after intubation. Following this, patients often experience an awareness of loss of physiological and personal autonomy and a feeling that someone or something is controlling them.
Conclusions
NPPV Plus UMC Versus UMC for First-Line Treatment of Acute Respiratory Failure Due to Acute Exacerbations of COPD
Moderate quality evidence showed that NPPV plus UMC significantly reduced the need for endotracheal intubation, inhospital mortality, and mean length of hospital stay compared with UMC.
Low quality evidence showed a lower rate of complications in the NPPV plus UMC group compared with the UMC group.
NPPV Versus IMV for Treatment of Acute Respiratory Failure in Patients Who Have Failed UMC
Because of inconsistent and low to very low quality evidence, there was insufficient evidence to draw conclusions on the comparison of NPPV versus IMV for patients who have failed medical treatment.
NPPV for Weaning COPD Patients from IMV
Moderate quality evidence showed that weaning COPD patients from IMV using NPPV results in significant reductions in mortality, nosocomial pneumonia, and weaning failure compared with weaning with IMV.
Low quality evidence showed a nonsignificant reduction in mean LOS and mean duration of mechanical ventilation in the NPPV group compared with the IMV group.
NPPV for Treatment of Recurrent Acute Respiratory Failure in COPD Patients After Extubation from IMV
Low quality evidence showed a nonsignificant reduction in rate of reintubation in the NPPV group compared with the UMC group; however, there was inadequate evidence to draw conclusions on the effectiveness of NPPV for the treatment of acute respiratory failure in COPD patients after extubation from IMV.
7. Noninvasive Positive Pressure Ventilation for Chronic Respiratory Failure
Background
In addition to its use in acute respiratory failure (described above), NPPV can be used to treat chronic respiratory failure in stable COPD patients.
In Ontario, ventilatory devices and positive airway pressure systems are covered under Respiratory Products by the Ministry of Health and Long-Term Care’s Assistive Devices Program. There are no specific guidelines for eligibility, but applicants must be assessed by a medical professional. According to the ventilator equipment pool database, there were 263 patients registered with a primary or secondary diagnosis of chronic bronchitis, emphysema, bronchiectasis, and chronic airway obstruction between 2005 and 2010. This may be an underestimate, because certain diagnoses, such as respiratory failure/respiratory insufficiency or hypoventilation, are not captured in the ventilator equipment pool.
Research Question
What is the effectiveness and cost-effectiveness of NPPV, compared with no ventilation while receiving usual care, for stable COPD patients?
Included Studies
As shown in Figure 9, of the 2,593 citations identified, 10 studies met the inclusion/exclusion criteria: 2 systematic reviews and 8 RCTs.
Figure 9: NPPV for Chronic Respiratory Failure Citation Flow Chart*.
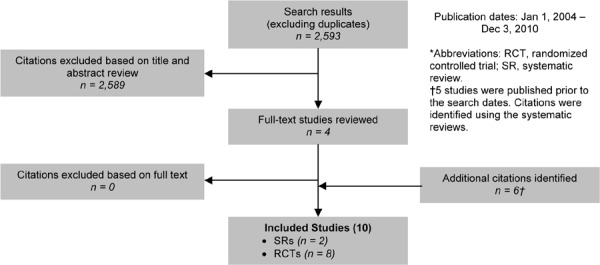
The 8 RCTs included a total of 403 participants. The sample size ranged from 13 to 144 participants. The mean age of the participants was 67 years of age. Based on either the GOLD COPD stage criteria or the mean percent predicted FEV1, 3 of the studies included people with severe COPD and 5 included people with very severe COPD.
All of the studies enrolled patients with stable COPD. Six of the trials were conducted in the outpatient setting and 2 in laboratory-based settings. Patients in the NPPV group received BiPAP ventilatory support with inspiratory levels set between 10 and 18 cm H2O. The amount of ventilation varied, ranging from 2 hours per day to 9 hours during the night. Usual care varied, but often included bronchodilators and LTOT.
The individual quality of the studies varied. Common methodological issues included lack of allocation concealment, lack of information on randomization methods, lack of blinding, limited generalizability (e.g., for studies conducted in a laboratory setting), and sparse data.
Results
Mortality
Three studies reported long-term (≥ 3 months) mortality. The pooled results showed a nonsignificant reduction in mortality in the NPPV group compared with the usual care group (RR, 0.89; 95% CI, 0.69–1.15; P = 0.39).
GRADE: moderate
Lung Function
Short term
One study reported mean change in percent predicted FEV1. The study showed a nonsignificant improvement in percent predicted FEV1 in the NPPV group compared with the usual care group (WMD, 5.00%; 95% CI, –1.91% to 11.91%; P = 0.16).
GRADE: very low
Long term
Two studies reported mean change in percent predicted FEV1. The pooled results showed a nonsignificant improvement in the NPPV group compared with the usual care group (WMD, 1.05%; 95% CI, –2.17% to 4.27%; P = 0.52).
GRADE: moderate
Exercise Capacity
Short term
Three studies reported functional exercise capacity assessment based on the 6MWT. The pooled results showed a clinically and statistically significant improvement in the NPPV group compared with the usual care group (WMD, 49.72 m; 95% CI, 2.93–96.51 m; P = 0.04).
GRADE: low
Long term
One study reported functional exercise capacity assessment based on the 6MWT. The study showed a nonsignificant decrease in exercise capacity in the NPPV group compared with the usual care group (WMD, –3.00 m; 95% CI, –52.55 m to 46.55 m; P = 0.91).
GRADE: moderate
Dyspnea
Four studies reported dyspnea. The results could not be pooled because different measures and characterizations of breathlessness were reported. Overall, there was a beneficial effect of NPPV as measured by the Borg Scale and Medical Research Council Score.
GRADE: low
Health-Related Quality of Life
Two studies reported HRQOL based on the SGRQ. The results of these studies could not be pooled because of insufficient data in the published reports. Overall, data on which to base a conclusion on the impact of NPPV on HRQOL were insufficient.
GRADE: n/a
Hospitalizations
Two studies reported short-term and long-term hospitalizations. Overall, there was no significant difference between the NPPV and usual care groups.
GRADE: moderate
Economic Model
Due to the lack of evidence of clinical effectiveness of NPPV in chronic respiratory failure in stable COPD, the results were not included in the economic model.
Experiences Concerning Ventilation (Qualitative Review)
The qualitative review of literature on noninvasive ventilation was not separated by acute or chronic respiratory failure. Thus, the results on patients’ perspectives on noninvasive ventilation are the same as those summarized in Noninvasive Positive Pressure Ventilation for Acute Respiratory Failure (see page 61 of this report).
Conclusions
Moderate quality evidence showed nonsignificant differences in mortality, lung function after 3 months, functional exercise capacity (6MWT) after 3 months, and hospitalizations between the NPPV and usual care groups.
Low quality evidence showed clinically and statistically significant improvements in functional exercise capacity (6MWT) for the first 3 months of treatment and a beneficial impact on dyspnea in the NPPV group compared with the usual care group.
There was insufficient evidence to draw conclusions about the impact of NPPV on HRQOL.
8. Hospital-at-Home Programs for Acute Exacerbations of COPD
Background
Hospital-at-home programs are services that provide patients with active treatment by health care professionals in the patient’s home for a condition that otherwise would require acute hospital inpatient care for a limited time period. Based on the programs described in the literature, when enrolled in hospital-at-home programs for COPD exacerbations, patients receive visits in their home from medical professionals (typically specialist nurses) who monitor the patients, alter their treatment plans if needed, and in some programs, provide additional care such as pulmonary rehabilitation, patient and caregiver education, smoking cessation counselling, and support services.
There are 2 types of hospital-at-home programs: admission avoidance and early discharge. In admission avoidance, after being assessed in the ED, patients are prescribed the necessary medications and additional care needed (e.g., oxygen therapy) and then sent home, where they will receive visits from medical professionals. Alternatively, in some programs patients may be referred directly to admission avoidance by their general practitioner, rather than first going to the ED. In early discharge, after being assessed in the ED, patients are admitted to the hospital where they receive the initial phase of their treatment, following which they are discharged early into hospital-at-home before the exacerbation has resolved. In both cases, once the exacerbation has resolved, the patient is discharged from the hospital-at-home program and no longer receives visits at home.
Hospital-at-home programs differ from other home care programs in 2 ways. First, they deal with patients who require higher-acuity care; in this case, patients have severe acute exacerbations of COPD and would otherwise require hospitalization for the treatment of their exacerbation. Second, hospitals retain the medical and legal responsibility for patients (at least in the models for COPD that have existed to date). Furthermore, patients requiring home care services may require these services for long periods of time or indefinitely, whereas patients in hospital-at-home programs require and receive the services for a limited period of time only (e.g., only until the acute exacerbation has resolved).
Hospital-at-home care is not appropriate for all patients with acute exacerbations of COPD. Those patients with less severe exacerbations who can be managed without admission to hospital are not eligible for hospital-at-home care. This includes patients who do not present to the ED for their exacerbation or those who can be discharged with some changes in medication only. Furthermore, some patients require admission to the hospital and cannot be safely treated in a hospital-at-home program, whether for medical reasons (e.g., diminished consciousness) or lack of/poor social support at home.
The proposed potential benefits of hospital-at-home for exacerbations of COPD include decreased health care resource utilization and decreased costs by avoiding hospital admissions and/or reducing LOS in the hospital; increased HRQOL for patients and their caregivers; and reduced risk of hospital-acquired infections in this susceptible, elderly, sick patient population.
Research Question
What is the effectiveness, cost-effectiveness, and safety for hospital-at-home care compared with inpatient hospital care of acute exacerbations of COPD?
Included Studies
As shown in Figure 10, of the 3,142 citations identified, 13 studies met the inclusion/exclusion criteria: 1 HTA, 5 systematic reviews, and 7 RCTs.
Figure 10: Hospital-at-Home for Acute Exacerbations Citation Flow Chart*.
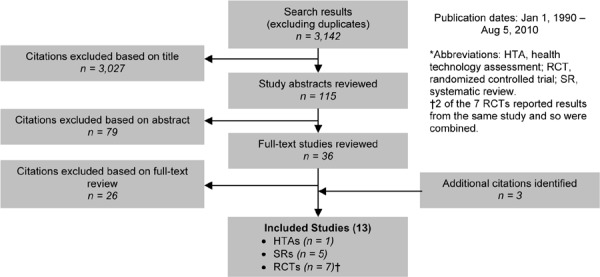
The 6 RCTs15 included a total of 611 patients. Sample size ranged from 32 to 184 people. The mean age of the participants ranged from about 66 to 80 years of age. Based on either the GOLD COPD stage criteria or the mean percent predicted FEV1, 3 of the studies included people with severe COPD. The other 3 studies could not be classified by severity of disease, as the necessary information was not provided.
Three studies used early discharge programs, 2 used admission avoidance programs, and 1 used both types of programs. The setting for hospital-at-home programs differed between study arms. In all studies, the control arm received care in the hospital. In the admission avoidance studies, patients in the hospital-at-home group received care in the home; in the early discharge studies, patients received some of their care in the hospital followed by early discharge and care in the home. Hospital-at-home programs varied between low-intensity programs (2 studies), in which patients were primarily monitored and received changes to medications as needed, and high-intensity programs (3 studies) that included additional care in the home such as pulmonary rehabilitation, social support services, COPD education, and smoking cessation counselling. One study did not provide adequate information for classification. Nurses made the home visits in all the studies, but in 1, doctors also made visits to the patients’ homes. The control group received usual care in hospital for the treatment of acute exacerbations of COPD.
The individual quality of the studies varied. Common methodological issues included not conducting analyses using intention-to-treat, lack of blinding and allocation concealment, and small sample sizes.
Results
Mortality
Six studies reported mortality from 2 to 6 months of follow-up. The pooled results showed a nonsignificant reduction in mortality in the hospital-at-home group compared with the inpatient hospital group (RR, 0.68; 95% CI, 0.41–1.12; P = 0.13). Subgroup analyses showed a significant reduction in mortality at 2 months of follow-up (RR, 0.32; 95% CI, 0.11–0.93; P = 0.04) and for the early discharge programs (RR, 0.33; 95% CI, 0.13–0.85; P = 0.02), but nonsignificant results for all other subgroups (3 and 6 months of follow-up [P = 0.91 and P = 0.47, respectively], admission avoidance programs [P = 0.63] and high-intensity [P = 0.71] and low-intensity [P = 0.26] programs).
GRADE: very low
Hospital Readmissions
Six studies reported hospital readmissions during follow-up. The pooled results showed a nonsignificant reduction in hospital readmissions in the hospital-at-home group compared with the inpatient hospital group (RR, 0.90; 95% CI, 0.70–1.16; P = 0.41). Subgroup analyses showed a significant reduction at 6 months of follow-up (RR, 0.59; 95% CI, 0.40–0.87; P = 0.009), but no other significant differences.
GRADE: low
Two studies showed an increase in mean days to readmission in the hospital-at-home group, which was significant in 1 of the studies (29.6 days vs. 25.6 days and mean [SD] 75 [55] days vs. 37 [29] days).
Thirteen to 50% of readmissions occurred early—that is, during the time that the patient was receiving visits in the home—with a weighted mean of 24.6%. However, not all readmissions occurred due to worsening of the patients’ condition.
Lung Function
Three studies reported lung function. The results could not be pooled because of different outcomes and methods of measurement. The studies that compared the mean change in FEV1 found no significant differences between the hospital-at-home and inpatient hospital groups.
GRADE: very low
Health-Related Quality of Life
Five studies reported HRQOL results using a variety of measures, including the SGRQ, CRQ, and the Geriatric Depression Scale. Overall, no significant differences were observed between the hospital-at-home and inpatient groups, with the exception of a significant improvement in the Geriatric Depression Scale and the Nottingham Health Profile score in the hospital-at-home group compared with the inpatient group in 1 of the studies. In 4 of the 5 studies, however, HRQOL was compared between baseline and end of follow-up, rather than during the time of the hospital-at-home or inpatient care program, and it may have been more appropriate to measure HRQOL results during the actual program.
GRADE: very low
Length of Stay
Six studies reported LOS, but the results could not be pooled due to differences in measurement. Three studies reported longer LOS in the hospital-at-home group, 1 reported a shorter LOS in the hospital-at-home group, and 1 reported similar LOS. Many of the hospital-at-home programs did not visit patients every day, which may inflate the LOS in these groups.
GRADE: very low
Patient Preference
One study reported patient and caregiver preference with the care as measured during the hospital-at-home and inpatient care. Patients receiving hospital-at-home care significantly preferred hospital-at-home care to inpatient care compared with patients receiving inpatient care (96.3% of patients in the hospital-at-home group preferred hospital-at-home care vs. 59.3% of patients in the inpatient hospital group preferred hospital-at-home care; P = 0.001). The same trend was observed for caregivers. These results, however, do not provide information on patients’ preference for hospital-at-home care before they have been enrolled in a program.
GRADE: very low
Satisfaction with Care
Three studies measured patient satisfaction with care and 1 measured caregiver satisfaction with care. Overall, satisfaction with care was very high for patients and caregivers in both hospital-at-home and inpatient care groups, and none of the studies observed a significant difference between the 2 groups.
GRADE: very low
Transfer to Long-Term Care
One study reported the number of patients who require transfer from home to long-term care after the acute exacerbation. A nonsignificant reduction in transfers to long-term care was observed in the hospital-at-home group compared with the inpatient care group (0 of 52 patients vs. 6 of 52 patients).
GRADE: very low
Eligibility for Hospital-at-Home Programs
Hospital-at-home programs are not appropriate for all patients with acute exacerbations of COPD. Eligibility criteria varied by study, but common reasons for excluding patients were absence of or poor home/social support, severe acidosis or alkalosis, severe comorbidities (e.g., cancer, dementia, renal failure, etc.), and acute chest radiograph changes. Overall, between 20.7% and 36.7% of patients who presented to EDs with acute exacerbations of COPD were enrolled in the included studies, though these estimates may underestimate the true number of eligible patients because of study-specific factors such as small geographic enrolment areas and the requirement to participate in a RCT.
Economic Model
Due to the low/very low quality of evidence and nonsignificant results, the hospital-at-home analysis was not included in the economic model.
Experiences with Hospital-at-Home Programs (Qualitative Review)
The qualitative literature search identified 24,906 citations of which 218 full-text studies were reviewed. Three studies related to early discharge or hospital-at-home programs in COPD patients. All 3 studies were conducted in primary care organizations in the United Kingdom. Several key themes emerged. First, COPD patients may often be unaware of early discharge schemes. Second, patients may be afraid of being discharged too early, whether they are part of an early discharge program or not. They may want—but nevertheless find it difficult—to negotiate the timing of their own hospital discharge. Third, transportation and medical dispensing can complicate discharge planning. Fourth, patients’ feelings about being at home varies: some may be glad to be home in familiar surroundings and appreciate responsive help via the telephone, but many find it difficult to resume necessary activities at home and fear future exacerbations or being alone. Some patients appreciate nurse home visits, while others find them unnecessary. Finally, patients may be averse to seeking medical help for problems that arise after going home, for fear of bothering health care providers or being readmitted to the hospital or in the hope that a problem will resolve itself.
Conclusions
Low quality evidence showed no significant differences in hospital readmissions between the hospital-at-home and inpatient care groups, but days to hospital readmission were increased in the hospital-at-home group compared with the inpatient care group.
Very low quality evidence showed no significant differences in mortality, HRQOL, or patient and caregiver satisfaction with care between the hospital-at-home and inpatient care groups.
There was insufficient evidence to determine the impact of hospital-at-home compared with inpatient care on lung function and LOS.
9. Home Telehealth
Background
Definitions for telehealth vary. For the purposes of this review, the following were used:
Telemedicine (or telehealth) refers to using advanced information and communication technologies and electronic medical devices to support the delivery of clinical care, professional education, and health-related administrative services. While telemedicine is often associated with direct patient clinical services, telehealth is often associated with a broader definition of remote health care and perceived to be more focused on other health-related services.
Telemonitoring (or remote monitoring) refers to using medical devices to remotely collect a patient’s vital signs and/or other health data and transmit those data to a monitoring station for interpretation by a health care provider.
Telephone-only support refers to disease/disorder management support provided by a health care provider to a patient’s residence via telephone or videoconferencing technology without transmitting patient biological data.
Telenursing generally refers to the regular, in-person visit of a health care provider, typically a nurse, to a patient’s residence to provide clinical care or professional education. Because of the resource requirements, telenursing is generally not feasible from a population perspective and is therefore not discussed further in this review.
In terms of telemonitoring, 2 types of devices are used: i) upload devices are wireless or modem-compatible devices that can measure biological information and directly upload the data either automatically or through patient assistance via landline or wireless transmission; and ii) entry devices are devices (either landline-based or wireless) or websites through which patients enter biological health data that was measured by a distinct measurement device. The monitoring of patient data by a health care practitioner can occur either in real time (i.e., real-time monitoring or synchronous monitoring) or can be stored and viewed at a later time (i.e., store-and-forward monitoring or asynchronous monitoring).
Because of the chronic nature of COPD and the subsequent need for continuous patient management, home telehealth technologies are increasingly being used to help outpatients maintain their independence and continue living in their own homes while ensuring their symptoms, vital signs, medication, education, and other management-related factors are monitored and/or managed and/or improved. There are 4 broad functions of home telehealth interventions for COPD:
to monitor vital signs or biological health data (e.g., oxygen saturation);
to monitor symptoms, medication, or other nonbiological endpoints (e.g., exercise adherence);
to provide information (education) and/or other support services (such as reminders to exercise or positive reinforcement); and
to establish a communication link between patient and health care provider.
These functions often require distinct technologies, although some devices can perform a number of these functions.
Research Questions
What is the effectiveness, cost-effectiveness, and safety of home telemonitoring compared with usual care for patients with COPD?
What is the effectiveness, cost-effectiveness, and safety of telephone-only support programs compared with usual care for patients with COPD?
Included Studies
As shown in Figure 11, of the 759 citations identified, 9 studies met the inclusion/exclusion criteria: 1 HTA, 1 systematic review, 5 RCTs, and 2 clinical controlled trials.
Figure 11: Telehealth for COPD Citation Flow Chart*.
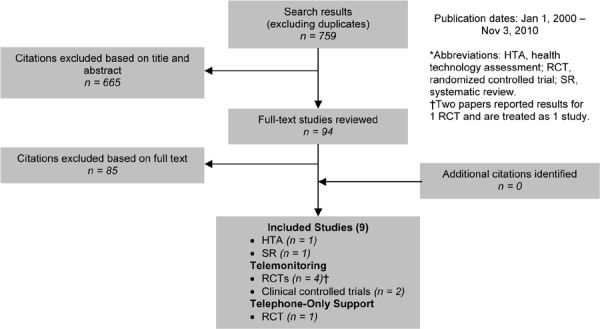
Results
Home Telemonitoring
The 516 trials (3 RCTs and 2 clinical controlled trials) included a total of 310 patients. The sample size ranged from 29 to 101 people. The mean age of the study participants ranged from 61 to 75 years. Based on either the GOLD COPD stage criteria or the mean percent predicted FEV1, trials varied in terms of disease severity of COPD participants. Two trials included patients with severe or very severe COPD, 1 included patients with moderate or severe COPD, 1 included patients with severe COPD only, and 1 included patients with moderate COPD only.
In the RCTs, the patients were randomized to receive either home telemonitoring or usual care, and in the clinical controlled trials, the patients or health care centres were non-randomly assigned to home telemonitoring or usual care. Three trials initiated telemonitoring following discharge from hospital, 1 following a pulmonary rehabilitation program, and 1 during management of patients at an outpatient clinic.
The home telemonitoring intervention involved measuring biological data such as oxygen saturation (i.e., pulse oximetry), FEV1, peak expiratory flow, and temperature. The telemonitoring devices varied: 3 of the 5 trials used an electronic health hub that performed multiple functions beyond the monitoring of biological parameters (e.g., it may have an electronic questionnaire for measuring symptoms), 1 trial used a pulse oximeter device connected to a modem, and 1 trial had patients measure and forward data to a nurse during televideo consultations. Usual care varied considerably between studies but often included follow-up care by a patient’s treating physician.
There was considerable clinical heterogeneity between trials in study design, methods, and intervention/control. The individual quality of the studies varied between studies. Common methodological issues included a lack of blinding, unplanned subgroup analyses, differences in important baseline variables between intervention and control, and a potential lack of power.
Hospitalizations
All-Cause
Four studies reported hospitalizations. Since hospitalizations were defined and measured differently across the trials, the data could not be pooled. The results were inconsistent. Three studies showed a nonsignificant reduction in hospitalizations for the home telemonitoring group compared with the usual care group; 2 studies, however, showed a significant reduction in hospitalizations.17 The study that was powered to assess hospitalizations did not find a significant difference between the 2 groups.
COPD-Specific
One trial reported COPD-specific hospitalizations. This study showed a nonsignificant reduction in hospitalizations in the home telemonitoring group compared with the usual care group (mean number of hospitalizations over 6 months: 0.20 vs. 0.35; P = 0.16). This study was powered to assess this outcome.
GRADE: very low (COPD-specific and all-cause hospitalizations combined)
Time Free of Hospitalizations
Two studies reported time free of hospitalizations as a secondary outcome. The results could not be pooled. The RCT showed a significant increase in time free of hospitalizations in the home telemonitoring group compared with the usual care group based on a Kaplan–Meier survival analysis adjusting for using home mechanical ventilation (P < 0.001). The clinical controlled trial also showed a protective benefit in the home telemonitoring group based on a multivariate Cox regression model adjusted for a number of factors including age and current smoking status (hazard ratio, 0.25; 95% CI, 0.09–0.60; P < 0.05).
GRADE: low
Mortality
One study evaluated mortality. The RCT showed no significant difference in mortality between the home telemonitoring group and usual care (P = 0.148), but no data were provided.
GRADE: low
Health-Related Quality of Life
Two studies reported the results of HRQOL. The results could not be pooled. One study showed a statistically and clinically significant improvement in mean change in total score for the SGRQ in the home telemonitoring group compared with the usual care group. While the mean change in SGRQ domain scores were also improved in the home telemonitoring group compared with usual care, these differences were not statistically significant. The second study showed no significant differences in HRQOL between the groups as measured by mean change in the total SGRQ score, hospital anxiety score, and EQ-5D. This study, however, was not powered to assess HRQOL.
GRADE: low
Length of Stay
Two studies reported hospital LOS as a secondary outcome. The results could not be pooled. Neither study showed a significant difference in median days in hospital between the home telemonitoring and usual care groups.
GRADE: low
Exacerbations
Two studies reported exacerbations as a secondary outcome. The results could not be pooled. The clinical controlled trial showed no significant difference in number of exacerbations (P > 0.05) between the home telemonitoring and usual care groups. The RCT showed a longer time until first exacerbation in a Kaplan–Meier survival analysis adjusting for using home mechanical ventilation in the home telemonitoring group compared with the usual care group (P < 0.001).
GRADE: low
Emergency Department Visits
Three studies reported ED visits as a secondary outcome. The results could not be pooled. Two studies showed no difference between the home telemonitoring and usual care groups for median ED visits per patient or total ED visits. One study showed that compared with usual care, the home telemonitoring group was more likely to have a longer time until first ED visit in a Kaplan–Meier survival analysis adjusting for using home mechanical ventilation (P < 0.001).
GRADE: low
Patient Satisfaction
Four studies reported patient satisfaction. Overall, these studies showed that participants generally felt safer or more secure when using home telemonitoring, perceived that the intervention was beneficial, and reported being satisfied with the equipment.
GRADE: n/a18
Economic Model
Due to low/very low quality evidence and nonsignificant results for the model inputs, home telemonitoring was not included in the economic model.
Telephone-Only Support
A total of 60 patients were included in the 1 RCT identified for telephone-only support. The mean age of the participants was 73.6 years. Patients of all severities of COPD were enrolled in the study. Participants were recruited from the medical department of an acute-care hospital in Hong Kong. They began receiving follow-up after they had been discharge from hospital with a diagnosis of COPD. The telephone support consisted of 2 telephone calls between 10 and 20 minutes long, the first occurring between days 3 and 7 and the second between days 14 and 20. These calls were led by a nurse and involved a structured, individualized educational and supportive program that focused on 3 components: assessment, management options, and evaluation. The usual care group did not receive telephone follow-up.
Health-Related Quality of Life
The study measured HRQOL using the Chinese Self-Efficacy Scale and showed significant improvements in the overall score, as well as in the Physical Exertion and Weather or Environment domains, in the telephone-only support group compared with the usual care group. In a multiple regression model, the conditions of telephone follow-up (β, 0.33; 95% CI, 0.19–0.48; P = 0.001), attendance at a pulmonary rehabilitation program (β, 0.44; 95% CI, 0.6–0.72; P = 0.003), smoking (β, 0.34; 95% CI, 0.09–0.57; P = 0.009), and health care use (β, 0.27; 95% CI, –0.07 to 0.47; P = 0.008) were significant factors in predicting patient self-efficacy.
GRADE: low
Hospitalizations
There was no significant difference between the telephone-only support and usual care groups when comparing mean hospitalizations per patient during the study and follow-up period (P = 0.20).
GRADE: low
Length of Stay
There was no significant difference in the mean LOS for hospital readmissions between the telephone-only support and usual care groups (P = 0.40).
GRADE: low
Emergency Department Visits
There was a significant reduction in the mean number of ED visits in the telephone-only support group compared with the usual care group (mean [SD], 0.1 [0.3] vs. 0.4 [0.7]; P = 0.03).
GRADE: low
Economic Model
Due to the low quality of evidence and nonsignificant results for the model inputs, telephone-only support was not included in the economic model.
Experiences Concerning Home Telehealth (Qualitative Review)
The literature search identified 24,906 citations, of which 218 full-text studies were reviewed. Eight studies related to home telehealth. Included studies were heterogeneous with regard to the study population and the type and application of telehealth technology offered to patients. Only 2 studies focused exclusively on patients with COPD; the remainder included patients with other complex chronic conditions as well as COPD. The types of technologies studied included telephone help lines, automated telephone services (for medication reminders or health-related weather warnings), videophones, and remote diagnostic monitoring. The primary focus of the qualitative review was 5 studies that examined patients’ and health care providers’ experiences with remote diagnostic monitoring technology. The main themes identified in these studies are summarized here.
Remote diagnostic monitoring can improve self-management, autonomy, and feelings of security for some patients, which may reduce health care visits and the burdensome process of travelling for care. Although patients may accept the technology in their homes, some patients may find the equipment difficult to use or accommodate.
Health care providers recognize potential benefits in terms of reduced need for clinical visits, better continuity of care, and enhanced collaboration between health care providers, but they also have reservations about possible negative changes to their duties and roles, with potentially new sources of legal liability if the technology interferes with optimal care.
Targeting patients for telehealth care poses challenges for both secondary prevention (population health) and equity, as current practices (i.e., setting up the technology for high-risk patients post hospital discharge) tend to miss patients at earlier disease stages, as well as those who do not speak English.
Conclusions
Home Telemonitoring
Low quality evidence showed that the time free of exacerbations, time free of hospitalizations, and time to ED visits were significantly improved in the home telemonitoring group compared with the usual care group. However, no significant differences were observed in terms of the number of exacerbations and ED visits.
Low to very low quality evidence showed conflicting results for HRQOL and hospitalizations, with some studies showing significant benefits in the home telemonitoring group compared with the usual care group, and other studies showing no significant differences between the 2 groups.
Low quality evidence showed no significant differences in mortality and LOS between the home telemonitoring and usual care groups.
There is substantial clinical heterogeneity between the trials, and since home telemonitoring is largely dependent on local information technologies, infrastructure, and personnel, the generalizability of these findings may be low.
Telephone-Only Support
Low quality evidence showed a significant reduction in ED visits and a significant improvement in HRQOL measured by the Chinese Self-Efficacy Scale for the telephone-only support group compared with the usual care group.
Low quality evidence showed no significant differences in hospitalizations and hospital LOS between the telephone support group and the usual care group.
Due to concerns regarding the generalizability of these results, additional research is required.
10. Experiences of Living and Dying with COPD
Qualitative empirical studies (from social sciences and clinical and related fields) offer important insights into how many COPD patients experience their condition, their needs, and health care and interventions. Before diagnosis, patients experience the suboptimal health that clinicians might call “early COPD,” but patients know as their own “normal,” and not necessarily an illness. Many patients initially misunderstand terms such as COPD, chronic obstructive pulmonary disease, or exacerbation. Some people with COPD prefer fuller prognostic information, while others fear and avoid it. Smokers may not readily understand or agree with the idea that smoking caused or worsens their COPD. Those who believe the causal link may feel regret or shame. Some feel stigmatized by care providers who seem to blame them, and avoid health care for this reason. The diagnosis and nature of the condition come into focus over time, with personal experience and piecemeal information from various sources.
COPD patients experience alternating good days and bad days. A roller coaster pattern of ups and downs becomes apparent, and COPD becomes a way of life. Patients use many means—social, psychological, medical, organizational—to control what they can, and to cope with what they cannot. Economic hardship, comorbidities, language barriers, or low health literacy can make coping more difficult. For smokers, medical advice to quit can conflict with smoking as a tool for coping with the stress of living with COPD. A patient’s sense of what is normal, as well as his/her tolerance of health problems and interventions, evolves with the progression of the disease.
Patients may not always attribute repeated exacerbations to advancing disease, but rather, as temporary setbacks caused by activities, environmental factors, faltering self-management, or infection. Although some exacerbations may create episodes of great dependency, patients may not expect a decline to total dependency over time. Dependency is challenging and disruptive, and patients often yearn for others to “be there” for them during crises. However, informal social support and formal social services are difficult to establish around intermittent and emergent needs. Many aspects of the COPD experience—physical, pragmatic, social, and emotional—isolate patients from others while also increasing their need for social support. These same challenges can impair patients’ access to, or rapport with, their health care providers.
The experience of chronic COPD challenges bodily integrity, self-confidence, and self-esteem. Many patients describe feeling powerless, helpless, hopeless, sad, frustrated, angry, anxious, or irritable. The incapacitation of COPD threatens one’s very identity, and many patients grieve for their lost roles, activities, and productivity. They may seek new sources of meaning in their lives. Late in the disease, the severity, duration, or frequency of bad days leads patients to recognize a permanent decline in health. Even so, patients may still envision death from COPD to be off in the distant, unpredictable future. They hope to recover from each exacerbation, but also fear dying from suffocation or breathlessness during these crises. Palliative end-of-life care may not be anticipated prior to referral for such care. A palliative care referral can convey the demoralizing message that providers have “given up.”
Family caregivers’ challenges often echo COPD patients’ own challenges, including anxiety, uncertainty about the future, helplessness, powerlessness, depression, difficulties maintaining employment, loss of mobility and freedoms, strained relationships, and growing social isolation. They too ride an “emotional roller coaster” over the course of the disease, with its evolving demands on care giving.
11. Preference for Ventilation among COPD Patients
Background
HTAs are increasingly considering patient values and preferences. Incorporating systematic reviews on patient preferences is one way of achieving this goal. To explore the feasibility of such an approach, we conducted a systematic review of patient preferences for ventilation among patients with COPD.
Study Objectives
to explore and discuss the feasibility of including systematic literature reviews on patient preferences within HTAs
to develop an appropriate search strategy for finding quantitative research on patient preferences
to summarize the literature on patient preferences for ventilation among COPD patients
to discuss the advantages and disadvantages of including patient preference data within HTAs
Methods
Databases were searched for studies published in English from 1990 through March 4, 2011. Two independent reviewers identified studies based on title and abstract. Full articles were retrieved if a decision could not be made based on the abstracts.
Inclusion Criteria
study participants met criteria for COPD
results for COPD were reported separately
at least 1 of the study interventions included IMV and/or NPPV for the treatment of COPD
patient preferences were reported
the study was quantitative
the study was not based on a quality of life indicator
Results
Preferences for IMV
The proportion of COPD patients who reported a willingness to use IMV varied considerably across studies, as estimates ranged from about 12% to 77%. Studies that used decision aids to elicit preferences or that emphasized IMV as an indefinite life support rather than as a temporary modality produced lower estimates (< 50%).
Preferences for NPPV
The proportion of COPD patients who expressed a willingness to try NPPV varied from 67% to 96%.
Satisfaction With IMV and NPPV
Two studies explored the experiences of COPD patients who had received ventilation. However, both of these had very small sample sizes (n = 9 and n = 11).
Predicting Ventilation Preferences
The results from this systematic review indicate that it is difficult to predict which COPD patients are likely to choose ventilation. Study results revealed no consistent association between patient preferences and covariates such as age, sex, education, marital status, FEV1, depression index, or quality-of-life scores.
Patient Preferences Vary by Context
Patient preferences can vary depending on how the intervention is presented and described to the patient. One study showed how preferences for NPPV ranged from 76% to 96%, depending on whether the patient was provided with a verbal description, a photograph, or a demonstration. Another study showed how patient preferences can vary when they were asked about their choices under different hypothetical health states. Rejection of IMV was 31% for COPD patients’ current health state, but ranged from 84% to 94% when they were asked about IMV under situations of permanent coma, dementia, or being bed-bound.
Conclusions
A significant proportion of COPD patients were willing to forgo a potentially life-saving intervention, particularly when it was framed as an indefinite procedure.
COPD patients who were willing to forgo either IMV or NPPV could not be reliably predicted by known covariates (such as age, quality of life).
COPD patient preferences for ventilation were not stable, but varied depending on how the intervention was described. Many COPD patients also altered their preferences when asked to consider ventilation under different hypothetical health states.
A systematic review of the patient preference literature offers many insights. However, the process is time-consuming due to the heterogeneity of study designs, outcomes measures, and terminology.
Summary of Results
Based on the results from the systematic reviews and economic model, the Ontario Health Technology Advisory Committee identified 10 treatment strategies for which there was adequate clinical/patient, health system, and cost-effectiveness evidence to make recommendations. For the remaining 8 treatment strategies, there was substantial uncertainty regarding the clinical/patient and health system outcomes due to low or very low quality of evidence. The mean ICER and the budget impact for each of these 8 strategies are unknown; the strategies were not included in the economic model due to the quality of evidence. The clinical and economic results are summarized below in Table 3.
Table 3: Summary of Findings by Topic and Research Question*.
| Intervention | Comparator | Study Population | No. Studies (N) | Summary Findings | GRADE Quality of Evidence |
|---|---|---|---|---|---|
| INFLUENZA VACCINATIONS | |||||
| Research Question: What is the effectiveness, safety, and cost-effectiveness of influenza vaccination compared with no vaccination in COPD patients? | |||||
| Influenza vaccine | Placebo | COPD patients | 1 (125) | Influenza vaccination significantly reduced the risk of influenza-related ARIs compared with placebo. | HIGH |
| Influenza vaccination had no significant impact on influenza-related ARI hospitalizations and the need for mechanical ventilation compared with placebo. | LOW | ||||
| Influenza vaccinations significantly increased local adverse reactions, but there was no significant difference in systemic reactions compared with placebo. | LOW | ||||
|
Economic model
Excluded from model as appropriate inputs were not available in the literature. |
n/a | ||||
| PNEUMOCOCCAL VACCINATIONS | |||||
| Research Question: What is the effectiveness, safety, and cost-effectiveness of pneumococcal vaccination compared with no vaccination in COPD patients? | |||||
| Pneumococcal vaccine | Placebo | COPD patients | 1 (596) | Pneumococcal vaccination significantly reduced the risk of pneumococcal pneumonia compared with placebo, but there was no significant difference in incidence of global pneumonia, episodes of global pneumonia, first episode of CAP, or time to first episode of CAP between the groups. | HIGH |
| Pneumococcal vaccination had no significant impact on hospitalizations due to CAP, hospital LOS, mortality, or local or systemic adverse reactions compared with placebo. | LOW | ||||
|
Economic model
Excluded from model as appropriate inputs were not available in the literature. |
n/a | ||||
| SMOKING CESSATION | |||||
| Research Question: What is the effectiveness and cost-effectiveness of smoking cessation interventions compared with usual care for patients with COPD? | |||||
| SC counselling | Usual care | COPD patients who smoke | 2(501) | Intensive SC counselling (≥ 90 minutes) significantly increased abstinences rates compared with usual care, but there was no significant difference in abstinence between the minimal counselling (< 90 minutes) and usual care groups. | MODERATE |
| SC counselling plus pharmacology (NRT and/or antidepressant) | Usual care | COPD patients who smoke | 5 (6,802) | Intensive SC counselling (≥ 90 minutes) plus NRT significantly increased abstinences rates compared with usual care, but there was no significant difference in abstinence between the minimal counselling (< 90 minutes) plus NRT and usual care groups, the minimal counselling plus antidepressant and usual care groups, and the minimal counselling plus NRT plus antidepressant and usual care groups. | MODERATE (intensive SC counselling plus NRT) LOW (all other comparisons) |
| NRT | Placebo | COPD patients who smoke | 1 (183) | NRT significantly increased abstinence rates compared with placebo. | MODERATE |
| Antidepressant | Placebo | COPD patients who smoke | 2 (596) | Bupropion significantly increased abstinence rates compared with placebo; however, nortriptyline had no significant impact on abstinence compared with placebo. | MODERATE |
Economic model
|
n/a | ||||
| MULTIDISCIPLINARY CARE | |||||
| Research Question: What is the effectiveness and cost-effectiveness of multidisciplinary care compared with usual care (single-care provider) for the treatment of stable COPD? | |||||
| MDC (2 or more providers) | Usual care (1 provider) | Patients with stable COPD | 6 (1,370) | MDC significantly improved all-cause and COPD-specific hospitalizations and COPD-specific ED visits compared with usual care. | MODERATE |
| MDC significantly improved HRQOL compared with usual care. | LOW | ||||
| MDC significantly improved lung function at 1 year compared with usual care. | VERY LOW | ||||
| MDC had no significant impact on mortality and all-cause ED visits compared with usual care. | LOW / VERY LOW | ||||
|
Economic model
|
n/a | ||||
| PULMONARY REHABILITATION | |||||
| Research Question 1: What is the effectiveness and cost-effectiveness of pulmonary rehabilitation compared with usual care for patients with stable COPD? | |||||
| PR | Usual care | Patients with stable COPD | 17 (1,159) | PR clinically and statistically significant improved HRQOL and functional exercise capacity (6MWT) compared with usual care. | MODERATE |
|
Economic model
Excluded from model as appropriate inputs were not available in the literature. |
n/a | ||||
| Research Question 2: Does early pulmonary rehabilitation (within 1 month of hospital discharge) in people who had an acute exacerbation of COPD improve outcomes compared with usual (or no rehabilitation)? | |||||
| PR | Usual care | Patients within 1 month of discharge from hospital due to acute exacerbations of COPD | 5 (276) | PR within 1 month of hospital discharge after an acute exacerbation of COPD significantly reduced hospital readmissions and resulted in clinically significant improvements in HRQOL and functional exercise capacity compared with usual care. | MODERATE |
Economic model
|
n/a | ||||
| Research Question 3: Do maintenance or post-rehabilitation programs for people with COPD who have completed a pulmonary rehabilitation program improve outcomes compared with usual care in people with COPD? | |||||
| PR maintenance | Usual care | Patients after discharge from a pulmonary rehab program | 3 (295) | PR maintenance programs had no significant impact on HRQOL, hospital admissions and LOS in the hospital compared with usual care. | LOW |
| PR maintenance programs resulted in statistically significant but not clinically significant improvements in exercise capacity compared with usual care. | LOW | ||||
|
Economic model
Excluded from model as appropriate inputs were not available in the literature. |
n/a | ||||
| LONG-TERM OXYGEN THERAPY | |||||
| Research Question 1: What is the effectiveness, cost-effectiveness, and safety of LTOT compared with no LTOT in COPD patients with severe hypoxemia? | |||||
| LTOT (> 15 hours/day) | No LTOT therapy, usual care | COPD patients with severe hypoxemia | 4 (263) | LTOT resulted in a borderline significant reduction in mortality compared with no LTOT. | LOW |
| LTOT significantly improved FEV1 and HRQOL compared with no LTOT. | LOW/VERY LOW | ||||
| LTOT resulted in increased hospitalizations§ but no difference in hospital LOS compared with no LTOT. | LOW | ||||
Economic model
|
n/a | ||||
| Research Question 2: What is the effectiveness, cost-effectiveness, and safety of LTOT compared with no LTOT in COPD patients with mild-to-moderate hypoxemia? | |||||
| LTOT (> 15 hours/day) | No LTOT, usual care | COPD patients with mild-to-moderate hypoxemia | 4 (539) | LTOT had no significant impact on mortality compared with no LTOT. | LOW |
| LTOT had no significant impact on lung function (% predicted FEV1), endurance time, or dyspnea compared with no LTOT. | VERY LOW | ||||
|
Economic model
Excluded from the economic model because of very low quality of evidence for model input (FEV1). |
n/a | ||||
| NPPV FOR THE TREATMENT OF ACUTE RESPIRATORY FAILURE DUE TO ACUTE EXACERBATIONS OF COPD | |||||
| Research Question 1a: What is the effectiveness, cost-effectiveness, and safety of NPPV for the treatment of acute hypercapnic respiratory failure due to acute exacerbations of COPD compared with UMC? | |||||
| NPPV + UMC | UMC | COPD patients with acute respiratory failure due to AECOPD | 11 (1,000) | NPPV significantly reduced the risk of endotracheal intubation and IMV, inhospital mortality, and mean hospital LOS compared with UMC. | MODERATE |
| NPPV resulted in fewer complications compared with UMC. | LOW | ||||
Economic model
|
n/a | ||||
| Research Question 1b: What is the effectiveness, cost-effectiveness, and safety of NPPV for the treatment of acute hypercapnic respiratory failure due to acute exacerbations of COPD for patients who failed medical treatment compared with IMV? | |||||
| NPPV | IMV | COPD patients with acute respiratory failure who failed medical treatment‖ | 2 (205) | At this time, the data could not be pooled and the results were conflicting. | LOW/VERY |
| No conclusions can be drawn regarding the comparative effectiveness of NPPV and IMV for this patient population. | LOW | ||||
|
Economic model
Excluded from the economic model due to conflicting results in the clinical evidence. |
n/a | ||||
| Research Question 2a: What is the effectiveness, cost-effectiveness and safety of NPPV compared with IMV for weaning COPD patients from IMV? | |||||
| NPPV | Pressure support IMV | COPD patients being invasively ventilated who failed T-piece weaning trials | 2 (80) | NPPV resulted in significant reductions in mortality, nosocomial pneumonia, and weaning failure compared with pressure support IMV. | MODERATE |
| NPPV had no significant impact on LOS in the ICU and duration of mechanical ventilation compared with pressure support IMV. | LOW | ||||
Economic model
|
n/a | ||||
| Research Question 2b: What is the effectiveness, cost-effectiveness, and safety of NPPV compared with UMC for the prevention of acute respiratory failure in COPD patients after they have been extubated from IMV? | |||||
| NPPV | UMC | COPD patients after they have been extubated from IMV | 0 (0) | No evidence was identified to evaluate the use of NPPV after extubation of COPD patients from IMV. | n/a |
|
Economic model
Excluded from economic model due to lack of evidence. |
n/a | ||||
| Research Question 2c: What is the effectiveness, cost-effectiveness, and safety of NPPV compared with IMV for the treatment of acute respiratory failure in COPD patients after they have been extubated from IMV? | |||||
| NPPV | IMV | COPD patients who develop respiratory failure within 48 hours of extubation from IMV | 1 (23) | NPPV had no significant impact on the reintubation rate based on a post hoc subgroup analysis of 23 patients with COPD. | LOW |
| At this time, there is inadequate evidence to reach conclusions on the comparative effectiveness of NPPV and UMC for the treatment of COPD patients who have developed acute respiratory failure following extubation from IMV. | n/a | ||||
|
Economic model
Excluded from economic model due to lack of evidence. |
n/a | ||||
| NPPV FOR THE TREATMENT OF CHRONIC RESPIRATORY FAILURE IN STABLE COPD | |||||
| Research Question: What is the effectiveness and cost-effectiveness of NPPV compared with no ventilation while receiving usual care for stable COPD patients with chronic respiratory failure? | |||||
| NPPV | Usual care | Stable COPD patients with chronic respiratory failure | 8 (403) | NPPV had no significant impact on mortality, lung function after 3 months, functional exercise capacity (6MWT) after 3 months, and hospitalizations compared with usual care. | MODERATE |
| NPPV clinically and statistically significantly improved functional exercise capacity (6MWT) during the first 3 months of treatment and had a beneficial impact on dyspnea compared with usual care. | LOW | ||||
|
Economic model
Excluded from economic model due to lack of clinical effectiveness. |
n/a | ||||
| HOSPITAL-AT-HOME PROGRAMS FOR ACUTE EXACERBATIONS OF COPD | |||||
| Research Question: What is the effectiveness, cost-effectiveness, and safety of hospital-at-home care compared with inpatient hospital care for acute exacerbations of COPD? | |||||
| Early discharge and admission avoidance HaH programs | Inpatient hospital care | COPD patients presenting to the ED with acute exacerbations of COPD that require admission to hospital | 6 (611) | HaH had no significant impact on hospital readmissions, but the days to readmission were increased in the HaH group compared with inpatient care. | LOW |
| HaH had no significant impact on mortality, HRQOL, and patient/caregiver satisfaction with care compared with inpatient care. | VERY LOW | ||||
|
Economic model
Excluded from economic model due to low/very low quality of evidence and nonsignificant differences between groups. |
n/a | ||||
| HOME TELEHEALTH | |||||
| Research Question 1: What is the effectiveness, cost-effectiveness and safety of home telemonitoring compared with usual care for patients with COPD? | |||||
| Home telemonitoring | Usual care | COPD patients | 5 (310) | Home telemonitoring significantly improved time free of exacerbations, time free of hospitalizations, and time to ED visits, but had no significant impact on number of exacerbations or ED visits. | LOW |
| The impact of home telemonitoring on HRQOL and hospitalizations could not be determined due to conflicting results in the literature. | LOW/VERY LOW | ||||
| Home telemonitoring had no significant impact on mortality and LOS compared with usual care. | LOW | ||||
|
Economic model
Excluded from economic model due to very low quality of evidence for the model inputs and inability to pool data for hospitalizations. |
n/a | ||||
| Research Question 2: What is the effectiveness, cost-effectiveness, and safety of telephone-only support programs compared with usual care for patients with COPD? | |||||
| Telephone-only support | Usual care | COPD patients | 1 (60) | Telephone-only support significantly reduced ED visits and significantly improved HRQOL measured by the Chinese Self-Efficacy Scale compared with usual care. | LOW |
| Telephone-only support had no significant impact on hospitalizations and hospital LOS compared with usual care. | LOW | ||||
|
Economic model
Excluded from economic model due to the low quality of evidence and nonsignificant difference between groups. |
n/a | ||||
Abbreviations: 6MWT, 6 minute walking test; AECOPD, acute exacerbation of COPD; ARI, acute respiratory illness; CAP, community-acquired pneumonia; COPD, chronic obstructive pulmonary disease; ED, emergency department; FEV1, forced expiratory volume in 1 second; HaH, hospital-at-home; HRQOL, health-related quality of life; ICER, incremental cost-effectiveness ratio; ICU, intensive care unit; IMV, invasive mechanical ventilation; LOS; length of stay; LTOT, long-term oxygen therapy; MDC, multidisciplinary care; NPPV, noninvasive positive pressure ventilation; NRT, nicotine replacement therapy; PaO2, partial pressure of oxygen (in arterial blood); PR, pulmonary rehabilitation; SC, smoking cessation; UMC, usual medical care.
Ranges reflect the results of the one-way sensitivity analysis that was performed for multidisciplinary care and pulmonary rehabilitation.
Based on the most recent FHT data, the costs of MDC programs to manage COPD were estimated at $85 million in FY 2010, with projected future expenditures of up to $51 million for incident cases, assuming the base case cost of program. However, this estimate does not accurately reflect the current costs to the province because of lack of report by FHTs, lack of capture of programs outside this model of care by any data set in the province, and because the resource utilization and frequency of visits/follow-up phone calls were based on the findings in the literature rather than the actual FHT COPD management programs in place in Ontario. Therefore, MDC resources being utilized in the province are unknown and difficult to measure.
In this study, patients in the LTOT arm had severe COPD, while patients in the no LTOT comparison arm had mild/moderate COPD.
While it was clear in 1 study that the patients had first failed usual medical care, this was not clear in the second study although it has been assumed.
Ontario Health Technology Advisory Committee Recommendations
Based on the clinical and economic evidence summarized above, using the Ontario Health Technology Advisory Committee Decision Determinants (1), the Ontario Health Technology Advisory Committee made the following recommendations:
-
1. The Ontario Health Technology Advisory Committee (OHTAC) recommends that any provincial strategy on chronic obstructive pulmonary disease (COPD) address gaps in patient and public knowledge about this disease and its causes, management, and course. Effective interventions for improving lay understanding of COPD should be identified.*
*In implementing this recommendation, Health Quality Ontario should communicate regarding the inadequate public recognition of this disease with Public Health Ontario, which is working with Cancer Care Ontario on a blueprint for management of the burden of chronic disease in the province. The under-recognition of COPD extends to health professionals, and this should be communicated to relevant training bodies.
Recommendations Regarding Secondary Prevention
-
2. OHTAC recommends maximizing the use of pneumococcal and influenza vaccines in patients with COPD, ensuring that vaccination reflects the established guidelines and recommendations for immunization.
OHTAC recommends that any barriers to making the pneumococcal vaccine easily available through physician offices be removed, thereby making the pneumococcal vaccine more accessible to patients.
Other opportunities to optimize access to influenza and pneumococcal vaccines, including patients with acute exacerbations of COPD admitted to hospital, should be explored.
-
3. OHTAC strongly endorses evidence-based strategies aimed at encouraging smoking cessation in patients with COPD.
Intensive counselling (≥90 minutes) is the most effective and cost-effective strategy, and should continue to be encouraged.
OHTAC recommends that consideration be made to providing training programs to health care professionals involved in providing intensive counselling.
OHTAC recommends bupropion or nicotine replacement therapies for smoking cessation.
Recommendations Regarding Stable COPD
4. OHTAC recommends ongoing access to existing community-based multidisciplinary care for the management of moderate to severe stable COPD.
5. OHTAC recommends ongoing access to existing pulmonary rehabilitation for the management of moderate to severe COPD in stable patients.
6. OHTAC recommends that long-term oxygen therapy continue to be provided to COPD patients with severe resting hypoxemia (≤ 55 mm Hg).
7. OHTAC does not recommend the use of noninvasive positive pressure ventilation (NPPV) for chronic respiratory failure in stable COPD patients due to its lack of clinical effectiveness.
Recommendations Regarding Acute Exacerbations of COPD
8. OHTAC recommends the use of pulmonary rehabilitation in patients following an acute exacerbation (within 1 month of hospital discharge).
9. OHTAC recommends the use of NPPV as an adjunct to usual medical care as a first-line treatment for patients with acute respiratory failure due to acute exacerbations of COPD who do not require immediate access to invasive mechanical ventilation (IMV). NPPV should be made widely available, with appropriate support systems and human resources for this indication.
10. OHTAC recommends the use of NPPV to wean COPD patients who have failed spontaneous breathing tests following IMV.
11. OHTAC recommends that patient preferences regarding mechanical ventilation be sought prior to acute respiratory decompensation, and should serve as a guide for the provision of this service.
Recommendations Regarding Palliative Care for COPD
12. In making palliative care services available, the fluctuating physical, psychosocial, spiritual, and information needs should be considered, without necessarily forgoing acute care or hope of improvement during and following severe exacerbations.
Recommendations Regarding Opportunities for Further Research
There was insufficient evidence for OHTAC to make recommendations on the following COPD treatment strategies:
hospital-at-home for the treatment of acute exacerbations
pulmonary rehabilitation maintenance programs
home telemonitoring
telephone-only support
NPPV versus IMV for the treatment of acute respiratory failure in patients who have failed medical treatment
NPPV for recurrent respiratory failure (postextubation)
long-term oxygen therapy for mildto-moderate hypoxemia
-
13. Due to substantial uncertainty arising from low/very low quality evidence of effectiveness and cost-effectiveness, but the potential for important health system and/or patient/clinical benefits, OHTAC recommends field evaluations for:
pulmonary rehabilitation maintenance programs
telemonitoring
As regards telemonitoring, OHTAC recommends that an evaluation of the proposed Ministry Telehomecare Expansion Project in partnership with Infoway, the Ontario Telemedicine Network, and the Local Health Integration Networks, which will encompass monitoring of patients with COPD, be undertaken and reported back to OHTAC upon completion.
Prior to expanding access to multidisciplinary care and pulmonary rehabilitation, OHTAC recommends field evaluation to evaluate long-term impacts of effectiveness and cost-effectiveness, optimal delivery of programs, characterization of patients most likely to benefit from these programs, and a survey of existing services.
14. Any primary research endorsed by OHTAC will include outcomes relevant to patient needs and perspectives, including patient preference, if applicable.
Implementation Considerations
In order to optimize the translation of the above recommendations into practice and to ensure high quality care, the formation of a provincial COPD Expert Panel to advise the health system and the Ministry of Health and Long-Term Care through Health Quality Ontario should be considered. This Expert Panel, representing patient and provider interests, should also inform OHTAC in an ongoing way of additional evidentiary requirements to further shape the COPD strategy.
Furthermore, opportunities to align these OHTAC recommendations to current funding strategies should be sought.
Feedback through public engagement expressed interest in pursuing other components of LTOT (oxygen assessment clinics, ambulatory oxygen therapy, and personal oximeters), OHTAC has reflected on this and regards these topics as out of scope of the existing overall mega-analysis on COPD, but these comments have been forwarded to the Assistive Devices Program (ADP). Similarly, through public engagement, OHTAC was made aware of the fact that there is a wide gap between the true costs of LTOT and the current funding level through ADP. This has also been forwarded to ADP.
Glossary
- 6 Minute Walking Test (6MWT)
A measure of exercise capacity which measures the distance that a patient can quickly walk on a flat, hard surface in a period of 6 minutes. A widely used outcome measure in respiratory rehabilitation of patients with COPD.
- Acute exacerbation of chronic obstructive pulmonary disease (AECOPD)
A change in baseline symptoms that is beyond day-to-day variation, particularly increased breathlessness, cough, and/or sputum, which has an abrupt onset.
- Admission avoidance hospital-at-homeprogram
Treatment program for patients experiencing acute exacerbations of COPD which allows patients to receive treatment in their home and avoid admission to hospital. After patients are assessed in the emergency department for an acute exacerbation, they are prescribed the necessary medications and additional care needed (e.g., oxygen therapy) and then sent home where they receive regular visits from a medical professional until the exacerbation has resolved.
- Ambulatory oxygen therapy
Provision of oxygen therapy during exercise and activities of daily living for individuals who demonstrate exertional desaturation.
- Bilevel positive airway pressure (BiPAP)
A continuous positive airway pressure mode used during noninvasive positive pressure ventilation (see definition below) that delivers preset levels of inspiratory and expiratory positive airway pressure. The pressure is higher when inhaling and falls when exhaling, making it easier to breathe.
- Cost-effectiveness acceptability curve (CEAC)
A method for summarizing uncertainty in estimates of cost-effectiveness.
- Cor pulmonale
Right heart failure, as a result of the effects of respiratory failure on the heart.
- Dyspnea
Difficulty breathing or breathlessness.
- Early discharge hospital-at-home program
Treatment program for patients experiencing acute exacerbations of COPD which allows patients to receive treatment in their home and decrease their length of stay in hospital. After being assessed in the emergency department for acute exacerbations, patients are admitted to the hospital where they receive the initial phase of their treatment. These patients are discharged early into a hospital-at-home program where they receive regular visits from a medical professional until the exacerbation has resolved.
- Forced expiratory volume in 1 second (FEV1)
A measure of lung function used for COPD severity staging; the amount of air that can be forcibly exhaled from the lungs in the first second of a forced exhalation.
- Forced vital capacity
The amount of air that can be forcibly exhaled from the lungs after taking
- (FVC)
the deepest breath possible.
- Fraction of inspired oxygen (FiO2)
The percentage of oxygen participating in gas exchange.
- Hypercapnia
Occurs when there is too much carbon dioxide in the blood (arterial blood carbon dioxide > 45 to 60 mm Hg).
- Hypopnea
Slow or shallow breathing.
- Hypoxemia
Low arterial blood oxygen levels while breathing air at rest. May be severe (PaO2 ≤ 55 mm Hg), moderate (56 mm Hg ≤ PaO2 < 65 mm Hg), or mild-to-moderate (66 mm Hg < PaO2 ≤ 74 mm Hg).19
- Incremental cost-effectiveness ratio (ICER)
Ratio of the change in costs of a therapeutic intervention to the change in effects of the intervention compared to the alternative (often usual care).
- Intention-to-treat analysis (ITT)
An analysis based on the initial treatment the participant was assigned to, not on the treatment eventually administered.
- Invasive mechanical ventilation (IMV)
Mechanical ventilation via an artificial airway (endotracheal tube or tracheostomy tube).
- Long-term oxygen therapy (LTOT)
Continuous oxygen use for about 15 hours per day. Use is typically restricted to patients fulfilling specific criteria.
- Multidisciplinary care
Defined as care provided by a team (compared to a single provider). Typically involves professionals from a range of disciplines working together to deliver comprehensive care that addresses as many of the patient’s health care and psychosocial needs as possible.
- Nicotine replacement therapy (NRT)
The administration of nicotine to the body by means other than tobacco, usually as part of smoking cessation.
- Noninvasive positive pressure ventilation (NPPV)
Noninvasive method of delivering ventilator support (without the use of an endotracheal tube) using positive pressure. Provides ventilatory support through a facial or nasal mask and reduces inspiratory work.
- Partial pressure of carbon dioxide (PaCO2)
The pressure of carbon dioxide dissolved in arterial blood. This measures how well carbon dioxide is able to move out of the body.
- Partial pressure of oxygen (PaO2)
The pressure of oxygen dissolved in arterial blood. This measures how well oxygen is able to move from the airspace of the lungs into the blood.
- Palliative oxygen therapy
Use of oxygen for mildly hypoxemic or nonhypoxemic individuals to relieve symptoms of breathlessness. Used short term. This therapy is “palliative” in that treatment is not curative of the underlying disease.
- Pulmonary rehabilitation
Multidisciplinary program of care for patients with chronic respiratory impairment that is individually tailored and designed to optimize physical and social performance and autonomy. Exercise training is the cornerstone of pulmonary rehabilitation programs.
- Pulse oximetry
A noninvasive sensor, which is attached to the finger, toe, or ear to detect oxygen saturation of arterial blood.
- Quality-adjusted life-year (QALY)
A measure of disease burden that includes both the quantity and the quality of the life lived that is used to help assess the value for money of a medical intervention.
- Respiratory failure
Respiratory failure occurs when the respiratory system cannot oxygenate the blood and/or remove carbon dioxide from the blood. It can be either acute (acute respiratory failure, ARF) or chronic, and is classified as either hypoxemic (type I) or hypercapnic (type II) respiratory failure. Acute hypercapnic respiratory failure frequently occurs in COPD patients experiencing acute exacerbations of COPD.
- Short-burst oxygen therapy
Short-duration, intermittent, supplemental oxygen administered either before or after exercise to relieve breathlessness with exercise.
- Sleep apnea
Interruption of breathing during sleep due to obstruction of the airway or alterations in the brain. Associated with excessive daytime sleepiness.
- Smoking cessation
The process of discontinuing the practice of inhaling a smoked substance.
- Spirometry
The gold standard test for diagnosing COPD. Patients breathe into a mouthpiece attached to a spirometer which measures airflow limitation.
- SpO2
Oxygen saturation of arterial blood as measured by a pulse oximeter.
- Stable COPD
The profile of COPD patients which predominates when patients are not experiencing an acute exacerbation.
- Supplemental oxygen therapy
Oxygen use during periods of exercise or exertion to relieve hypoxemia.
- Telemedicine (or telehealth)
Refers to using advanced information and communication technologies and electronic medical devices to support the delivery of clinical care, professional education, and health-related administrative services.
- Telemonitoring (or remote monitoring)
Refers to the use of medical devices to remotely collect a patient’s vital signs and/or other biologic health data and the transmission of those data to a monitoring station for interpretation by a health care provider.
- Telephone only support
Refers to disease/disorder management support provided by a health care provider to a patient who is at home via telephone or videoconferencing technology in the absence of transmission of patient biologic data.
- Ventilator-associated pneumonia (VAP)
Pneumonia that occurs in patients undergoing mechanical ventilation while in a hospital.
Acknowledgements
Editorial Staff
Jan Collins
Joanna Odrowaz
Jeanne McKane
COPD Expert Advisory Panel
The role of the expert panel was to provide direction on the scope of the project and the relevant outcomes measures of effectiveness, to review the evidence-based analyses and to identify any societal or systemic issues that are relevant to intervention effectiveness. However, the statements, conclusions and views expressed in this report do not necessarily represent the views of the expert panel members.
Jeremy Grimshaw, MD, MBChB, PhD (Chair)
Senior Scientist, Ottawa Hospital Research Institute
Professor, Department of Medicine, University of Ottawa
Dina Brooks, PhD
Professor, Department of Physical Therapy, University of Toronto
Debbie Coutts, RRT, CRE
Andrea Gershon, MD, MSc, FRCP(C)
Scientist, Institute for Clinical Evaluative Sciences
Respirologist, Sunnybrook Health Sciences Centre
Assistant Professor, Departments of Medicine and Health Policy, Management and Evaluation, University of Toronto
Mita Giacomini, BSc, MPH, MA, PhD
Professor, Department of Clinical Epidemiology & Biostatistics, McMaster University
Ron Goeree, BA, MA
Director, PATH Research Institute, St. Joseph’s Hospital (Hamilton)
Associate Professor, Department of Clinical Epidemiology & Biostatistics, McMaster University
Roger Goldstein, MBCHB, FRCP(C), FRCP(UK)
NSA Chair in Respiratory Rehabilitation Research
Director, Respiratory Services, and Senior Scientist, West Park Healthcare Centre
Professor, Medicine and Physical Therapy, University of Toronto
Alan G Kaplan, MD, CCFP(EM), FCFP
Chairperson, Family Physician Airways Group of Canada
Chairperson, Special Interest Focused Care Group in Respiratory Medicine, College of Family Physicians of Canada
Clinical Lecturer, Department of Family and Community Medicine, University of Toronto
DE O’Donnell, MD, FRCP(C)
Director, COPD Centre, Kingston General Hospital
Professor, Department of Medicine, Queen’s University
Asad Razzaque, MD
Family Physician
Holger Schünemann, MD, PhD, MSc, FRCP(C)
Michael Gent Chair in Healthcare Research
Chair, Department of Clinical Epidemiology & Biostatistics, McMaster University
Professor, Department of Clinical Epidemiology & Biostatistics and Medicine, McMaster University
Tasnim Sinuff, MD, PhD, FRCP(C)
Clinician Scientist, Sunnybrook Health Sciences Centre
Assistant Professor, Department of Medicine, University of Toronto
Laura Watling, RRT, BSc(HK)
Clinical Practice Leader/Clinical Coordinator, Respiratory Therapy, West Park Healthcare Centre
Experiences of Living and Dying with COPD Report
This study was funded by the Canadian Institutes of Health Research, Grant # TOO-105430. Deirdre DeJean and Andrea Smith were supported by CIHR Frederick Banting and Charles Best Canada Graduate Scholarships (CGS) Doctoral Awards. We thank the Ontario Ministry of Health and Long-Term Care for its support for the Centre for Health Economics and Policy Analysis (CHEPA) at McMaster University, whose collegial environment made this project possible.
Appendices
Appendix 1: Scoping Search Strategies
Database: Ovid MEDLINE(R) <1996 to June Week 2 2010>
Search Strategy:
--------------------------------------------------------------------------------
exp Pulmonary Disease, Chronic Obstructive/ (13437)
(chronic obstructive adj2 (lung* or pulmonary or airway* or airflow or respiratory) adj (disease* or disorder*)).ti,ab. (14383)
(copd or coad).ti,ab. (12702)
chronic airflow obstruction.ti,ab. (110)
exp Emphysema/ (2872)
((chronic adj2 bronchitis) or emphysema).ti,ab. (8319)
or/1-6 (29151)
Database: EMBASE <1980 to 2010 Week 23>
Search Strategy:
--------------------------------------------------------------------------------
exp chronic obstructive lung disease/ (35645)
(chronic obstructive adj2 (lung* or pulmonary or airway* or airflow or respiratory) adj (disease* or disorder*)).ti,ab. (19275)
(copd or coad).ti,ab. (15657)
chronic airflow obstruction.ti,ab. (453)
exp emphysema/ (14476)
exp chronic bronchitis/ (6184)
((chronic adj2 bronchitis) or emphysema).ti,ab. (14524)
or/1-7 (58190)
OHTAC COPD Collaborative
Project Lead
BR McCurdy
Medical Advisory Secretariat (MAS)
BR McCurdy, M Bornstein, J Franek, K Kaulback, S Sehatzadeh, N Sikich, M Thabane, the COPD Working Group, and L Levin
Centre for Health Economics and Policy Analysis (CHEPA) at McMaster University
M Giacomini, D DeJean, D Simeonov, A Smith
Program for Assessment of Technology in Health (PATH) Research Institute
K Chandra, G Blackhouse, K Campbell, R Goeree
Toronto Health Economics and Technology Assessment (THETA) Collaborative
AS Brooker, SM Carcone, W Witteman, M Krahn
Suggested Citation
This report should be cited as follows:
OHTAC COPD Collaborative. Chronic obstructive pulmonary disease (COPD) evidentiary framework. Ont Health Technol Assess Ser [Internet]. 2012 March; 12(2):1-97. Available from: www.hqontario.ca/en/mas/tech/pdfs/2012/rev_COPD_Framework_March.pdf
Indexing
The Ontario Health Technology Assessment Series is currently indexed in Excerpta Medica/EMBASE and the Center for Reviews and Dissemination database.
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to: MASinfo@hqontario.ca.
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: http://www.hqontario.ca/en/mas/mas_ohtas_mn.html.
Conflict of Interest Statement
All analyses in the Ontario Health Technology Assessment Series are impartial and subject to a systematic evidence-based assessment process. There are no competing interests or conflicts of interest to declare.
Peer Review
All analyses in the Ontario Health Technology Assessment Series are subject to external expert peer review. Additionally, the public consultation process is also available to individuals wishing to comment on an analysis prior to finalization. For more information, please visit: http://www.hqontario.ca/en/mas/ohtac_public_engage_overview.html.
Health Quality Ontario
130 Bloor Street West, 10th Floor
Toronto, Ontario
M5S 1N5
Tel: 416-323-6868
Toll Free: 1-866-623-6868
Fax: 416-323-9261
Email: MASinfo@hqontario.ca
ISSN 1915-7398 (online)
ISBN 978-1-4435-7018-3 (PDF)
© Queen’s Printer for Ontario, 2012
About the Medical Advisory Secretariat
Effective April 5, 2011, the Medical Advisory Secretariat (MAS) became a part of Health Quality Ontario (HQO), an independent body funded by the Ministry of Health and Long-Term Care. The mandate of MAS is to provide evidence-based recommendations on the coordinated uptake of health services and health technologies in Ontario to the Ministry of Health and Long-Term Care and to the health care system. This mandate helps to ensure that residents of Ontario have access to the best available and most appropriate health services and technologies to improve patient outcomes.
To fulfill its mandate, MAS conducts systematic reviews of evidence and consults with experts in the health care services community. The resulting evidence-based analyses are reviewed by the Ontario Health Technology Advisory Committee—to which MAS also provides a secretariat function—and published in the Ontario Health Technology Assessment Series.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, MAS systematically reviews the available scientific literature, making every effort to consider all relevant national and international research; collaborates with partners across relevant government branches; consults with clinical and other external experts and developers of new health technologies; and solicits any necessary supplemental information.
In addition, the Secretariat collects and analyzes information about how a new technology fits within current practice and existing treatment alternatives. Details about the technology’s diffusion into current health care practices add an important dimension to the review of the provision and delivery of the health technology in Ontario. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social and legal issues relating to the technology assist decision-makers in making timely and relevant decisions to optimize patient outcomes.
The public consultation process is available to individuals wishing to comment on an analysis prior to publication. For more information, please visit: http://www.hqontario.ca/en/mas/ohtac_public_engage_overview.html.
Disclaimer
This evidence-based analysis was prepared by MAS for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation, and comparison of scientific research and/or technology assessments conducted by other organizations. It also incorporates, when available, Ontario data and information provided by experts and applicants to MAS to inform the analysis. While every effort has been made to reflect all scientific research available, this document may not fully do so. Additionally, other relevant scientific findings may have been reported since completion of the review. This evidence-based analysis is current to the date of the literature review specified in the methods section. This analysis may be superseded by an updated publication on the same topic. Please check the MAS website for a list of all evidence-based analyses: http://www.hqontario.ca/en/mas/mas_ohtas_mn.html.
List of Tables
List of Figures
| Figure 1: Prevalence of COPD in Ontario in 2006/2007 (Adults Aged 35 Years and Older)* |
| Figure 2: COPD Model Structure* |
| Figure 3: Influenza and Pneumococcal Vaccinations for COPD Citation Flow Chart* |
| Figure 4: Smoking Cessation for COPD Citation Flow Chart* |
| Figure 5: Multidisciplinary Care for COPD Citation Flow Chart* |
| Figure 6: Pulmonary Rehabilitation for COPD Citation Flow Chart* |
| Figure 7: Long-Term Oxygen Therapy for COPD Citation Flow Chart* |
| Figure 8: NPPV for Acute Respiratory Failure Citation Flow Chart* |
| Figure 9: NPPV for Chronic Respiratory Failure Citation Flow Chart* |
| Figure 10: Hospital-at-Home for Acute Exacerbations Citation Flow Chart* |
| Figure 11: Telehealth for COPD Citation Flow Chart* |
List of Abbreviations
- 6MWT
6 Minute Walking Test
- AECOPD
Acute exacerbation of COPD
- ARI
Acute respiratory illness
- BIA
Budget impact analysis
- BiPAP
Bilevel positive airway pressure
- CAP
Community-acquired pneumonia
- CEA
Cost-effectiveness analysis
- COPD
Chronic obstructive pulmonary disease
- CI
Confidence interval(s)
- CRQ
Chronic Respiratory Questionnaire
- ED
Emergency department
- FEV1
Forced expiratory volume in 1 second
- FHT
Family Health Team
- FVC
Forced vital capacity
- FY
Fiscal year
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- HaH
Hospital-at-home
- HRQOL
Health-related quality of life
- HTA
Health technology assessment
- IC
Intensive counselling
- ICER
Incremental cost-effectiveness ratio
- ICU
Intensive care unit
- IMV
Invasive mechanical ventilation
- LOS
Length of stay
- LTOT
Long-term oxygen therapy
- MAS
Medical Advisory Secretariat
- MCID
Minimal clinically important difference
- MDC
Multidisciplinary care
- NPPV
Noninvasive positive pressure ventilation
- NRT
Nicotine replacement therapy
- OHTAC
Ontario Health Technology Advisory Committee
- PaO2
Partial pressure of oxygen
- QALY
Quality-adjusted life-year
- RCT
Randomized controlled trial
- RR
Relative risk
- SC
Smoking cessation
- SD
Standard deviation
- SGRQ
St. George’s Respiratory Questionnaire
- SR
Systematic review
- UMC
Usual medical care
- VAP
Ventilator-associated pneumonia
- WMD
Weighted mean difference
Footnotes
Based on all-cause mortality data from Ontario administrative health data sets. (8)
Observational studies were included only in those reviews where the RCT evidence did not include results for important outcomes: that is, the LTOT and home telehealth reviews.
Two of the RCTs reported results from the same study; these papers were treated as 1 publication.
The minimal clinically important difference (MCID) in the SGRQ is 4 points. (31)
The MCID for the SGRQ is 4 points. (31)
The MCID for the CRQ is 0.5 units. (31)
The MCID for the SGRQ is 4 points. (31)
The MCID for the CRQ is 0.5 units. (31)
The MCID for the SGRQ is 4 points. (31)
Usual medical care is the term used for the medical treatment of patients with acute respiratory failure as an alternative to NPPV. Usual care is the generic term for the comparison group in other analyses.
Fourteen papers were identified; however 2 of the trials reported results for 1 study but different outcomes. These 2 papers have been treated as 1 study.
Two of the 7 RCTs reported the results for the same study and so were combined.
2 of the 5 total RCTs reported results of the same parent study and so were combined.
One study reported hospitalizations in 2 ways: proportion of patients with at least 1 hospitalization and mean number of hospitalizations over 6 months follow-up. These 2 results are counted separately here.
Outcomes of patient satisfaction were sparsely reported and their method of assessment varied widely, making it impossible to apply GRADE.
The mild-to-moderate classification was created for the purposes of the report.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease [Internet] 2010. [[cited: 2011 Jul 6]]. p. 97. [place unknown]: Global Initiative for Chronic Obstructive Lung Disease, Inc. Available from: http://www.goldcopd.org/uploads/users/files/GOLDReport_April112011.pdf .
- 2.O’Donnell DE, Hernandez P, Kaplan A, Aaron S, Bourbeau J, Marciniuk D, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease — 2008 update — highlights for primary care. Can Respir J. 2008. Jan 15, pp. 1A–8A. [DOI] [PMC free article] [PubMed]
- 3.Niewoehner DE. Clinical practice. Outpatient management of severe COPD. N Engl J Med. 2010 Apr 15;362(15):1407–16. doi: 10.1056/NEJMcp0912556. [DOI] [PubMed] [Google Scholar]
- 4.Donaldson GC, Wedzicha JA. COPD exacerbations. 1: Epidemiology. Thorax. 2006 Feb;1(2):164–8. doi: 10.1136/thx.2005.041806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hurst JR, Donaldson GC, Quint JK, Goldring JJ, Baghai-Ravary R, Wedzicha JA. Temporal clustering of exacerbations in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009 Mar 1;79(5):369–74. doi: 10.1164/rccm.200807-1067OC. [DOI] [PubMed] [Google Scholar]
- 6.Chapman KR, Bourbeau J, Rance L. The burden of COPD in Canada: results from the Confronting COPD survey. Respir Med. 2003 Mar;97(Suppl C):S23–S31. doi: 10.1016/s0954-6111(03)80022-7. [DOI] [PubMed] [Google Scholar]
- 7.Public Health Agency of Canada. COPD [Internet] [[updated 2007 Nov 22; cited 2011 Jun 21]]. [Ottawa (ON)] : Public Health Agency of Canada; Available from: http://www.phac-aspc.gc.ca/publicat/2007/lbrdc-vsmrc/copd-mpoc-eng.php .
- 8.Gershon AS, Wang C, Wilton AS, Raut R. To T. Trends in chronic obstructive pulmonary disease prevalence incidence, and mortality in Ontario Canada, 1996 to 2007: a population-based study. Arch Intern Med. 2010 Mar 22;170(6):560–5. doi: 10.1001/archinternmed.2010.17. [DOI] [PubMed] [Google Scholar]
- 9.Institute for Clinical Evaluative Sciences. Prevalence rate of chronic obstructive pulmonary disease (COPD) [Internet] [[updated 2011; cited 2011 Jun 12]]. Toronto (ON): Institute for Clinical Evaluative Sciences; Available from: http://intool.ices.on.ca/Default.aspx .
- 10.Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007 Sep 1;370(9589):765–73. doi: 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- 11.Devereux G. ABC of chronic obstructive pulmonary disease. Definition, epidemiology, and risk factors. BMJ. 2006 May 13;332(7550):1142–4. doi: 10.1136/bmj.332.7550.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Clinical Guideline Centre. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care [Internet] 2010. [[cited: 2011 Jun 6].]. p. 673. London (U.K.): National Clinical Guideline Centre. Available from: http://www.nice.org.uk/nicemedia/live/13029/49425/49425.pdf .
- 13.Editorial Board for Respiratory Disease in Canada. Respiratory disease in Canada [Internet] 2001. [[cited: 2011 Jun 12].]. p. 118. [Ottawa (ON)]: Health Canada. Available from: http://www.phac-aspc.gc.ca/publicat/rdc-mrc01/pdf/rdc0901e.pdf .
- 14.Statistics Canada. Leading causes of death [Internet] [[updated 2007; cited 2011 Jul 7]]. Ottawa (ON): Statistics Canada. Available from: http://www.statcan.gc.ca/daily-quotidien/101130/dq101130b-eng.htm .
- 15.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997 May 24;349(9064):1498–504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 16.Marciniuk DD, Goodridge D, Hernandez P, Rocker G, Balter M, Bailey P, et al. Managing dyspnea in patients with advanced chronic obstructive pulmonary disease: a Canadian Thoracic Society clinical practice guideline. Can Respir J. 2011 Mar;18(2):69–78. doi: 10.1155/2011/745047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medical Advisory Secretariat. Population-based smoking cessation strategies: a summary of a select group of evidence-based reviews. [[cited: 2011 Jun 12]];Ont Health Technol Assess Ser [Internet] 2010 Jan;10(1):1–44. Available from: http://www.hqontario.ca/en/mas/mas_ohtas_tech_smoking_20100120.html . [PMC free article] [PubMed] [Google Scholar]
- 18.Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007 Sep 1;370(9589):741–50. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 19.Hill K, Goldstein RS, Guyatt GH, Blouin M, Tan WC, Davis LL, et al. Prevalence and underdiagnosis of chronic obstructive pulmonary disease among patients at risk in primary care. CMAJ. 2010;182(7):673–8. doi: 10.1503/cmaj.091784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin K, Watkins B, Johnson T, Rodriguez JA, Barton MB. Screening for chronic obstructive pulmonary disease using spirometry: summary of the evidence for the U.S. Preventative Services Task Force [Internet] Rockville (MD): Agency for Healthcare Research and Quality; 2008. [[cited: 2011 Jun 12]. Report No. 59]. Available from: http://www.uspreventiveservicestaskforce.org/uspstf08/copd/copdes.pdf . [PubMed]
- 21.U.S. Preventative Services Task Force. Screening for chronic obstructive pulmonary disease using spirometry: recommendation statement [Internet] [[updated 2008; cited 2011 Jun 22]]. Available from: http://www.uspreventiveservicestaskforce.org/uspstf08/copd/copdrs.htm .
- 22.Meyers BF, Patterson GA. Chronic obstructive pulmonary disease. 10: Bullectomy, lung volume reduction surgery, and transplantation for patients with chronic obstructive pulmonary disease. Thorax. 2003 Jul;58(7):634–8. doi: 10.1136/thorax.58.7.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tiong LU, Gibson PG, Hensley MJ, Hepworth R, Lasserson TJ, Smith B, Davies HRHR. Lung volume reduction surgery for diffuse emphysema. Cochrane Database Syst Rev. 2006. Art. No.: CD001001. DOI: 10.1002/14651858.CD001001.pub2. [DOI] [PubMed]
- 24.Benditt JO. Surgical options for patients with COPD: sorting out the choices. Respir Care. 2006 Feb;51(2):173–82. [PubMed] [Google Scholar]
- 25.Snider GL. Reduction pneumoplasty for giant bullous emphysema. Implications for surgical treatment of nonbullous emphysema. Chest. 1996 Feb;109(2):540–8. doi: 10.1378/chest.109.2.540. [DOI] [PubMed] [Google Scholar]
- 26.Patel N, Criner GJ. Transplantation in chronic obstructive pulmonary disease. COPD. 2006 Aug;3(3):149–62. doi: 10.1080/15412550600829364. [DOI] [PubMed] [Google Scholar]
- 27.Martinez FJ, Chang A. Surgical therapy for chronic obstructive pulmonary disease. Semin Respir Crit Care Med. 2005 Apr;26(2):167–91. doi: 10.1055/s-2005-869537. [DOI] [PubMed] [Google Scholar]
- 28.Review Manager (RevMan) [Computer program] Version 5.1. Copenhagen (DK): The Nordic Cochrane Centre, The Cochrane Collaboration. 2011.
- 29.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004 Jun 19;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005 Feb 15;142(4):233–9. doi: 10.7326/0003-4819-142-4-200502150-00005. [DOI] [PubMed] [Google Scholar]
- 31.Puhan MA, Gimeno-Santos E, Scharplatz M, Troosters T, Walters EH, Steurer J. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011. Art. No.: CD005305. DOI: 10.1002/14651858.CD005305.pub3. [DOI] [PubMed]
- 32.Puhan MA, Mador MJ, Held U, Goldstein R, Guyatt GH, Schunemann HJ. Interpretation of treatment changes in 6-minute walk distance in patients with COPD. Eur Respir J. 2008;32(3):637–43. doi: 10.1183/09031936.00140507. [DOI] [PubMed] [Google Scholar]
- 33.Holland AE, Hill CJ, Rasekaba T, Lee A, Naughton MT, McDonald CF. Updating the minimal important difference for six-minute walk distance in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2010 Feb;91(2):221–5. doi: 10.1016/j.apmr.2009.10.017. [DOI] [PubMed] [Google Scholar]


