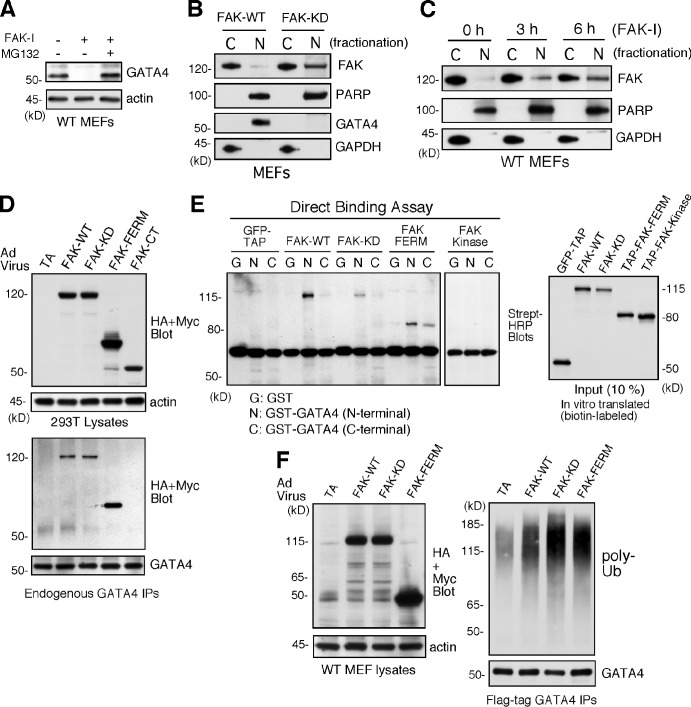
Figure 6.
Inhibited FAK is nuclear localized, and FAK-FERM binds GATA4 to promote GATA4 ubiquitination in cells. (A) FAK-WT MEFs treated with DMSO, FAK-I (1 µM PF271), or FAK-I with MG132 (40 µM) for 12 h and lysates blotted for GATA4 and actin. (B) FAK-WT and FAK-KD MEF lysates were separated into cytosolic (C) or nuclear (N) fractions and immunoblotted for FAK, PARP, GATA4, and GAPDH. PARP and GAPDH are nuclear and cytosolic markers, respectively. (C) FAK inhibition promotes FAK nuclear localization within 3 h. FAK-WT MEFs were treated with FAK-I (1 µM PF271) for the indicated times, and fractionated lysates were immunoblotted for FAK, PARP, and GAPDH. (D) 293T cells were transduced with Ad-TA, the indicated HA- or Myc-tagged FAK-WT, FAK-KD, FAK-FERM, and FAK-CT constructs, and association with endogenous GATA4 was determined by coimmunoprecipitation (IP). Immunoblotting shows expression of FAK constructs or actin (top) and FAK-FERM association with GATA4 (bottom). (E) GATA4 directly binds FAK. In vitro translated GFP–tandem affinity probe (TAP), FAK-WT, FAK-KD, TAP-FAK-FERM, and TAP-FAK kinase domain (386–686) were used in a direct binding assay with GST or GST fusions of GATA4 N terminus or GATA4 C terminus. Streptavidin (Strept)-HRP analyses show the amount of FAK bound (left) or 10% of input (right). (F) FAK-FERM enhances GATA4 ubiquitination. FAK-WT MEFs were transduced with Ad-TA, the indicated FAK constructs, and flag-tagged GATA4 and treated with MG132 (40 µM, 3 h). Whole-cell lysates were analyzed for expression of FAK or actin (left), and flag tag immunoprecipitates (antibody coupled to beads) were evaluated by anti-ubiquitin (Ub) and GATA4 immunoblotting.