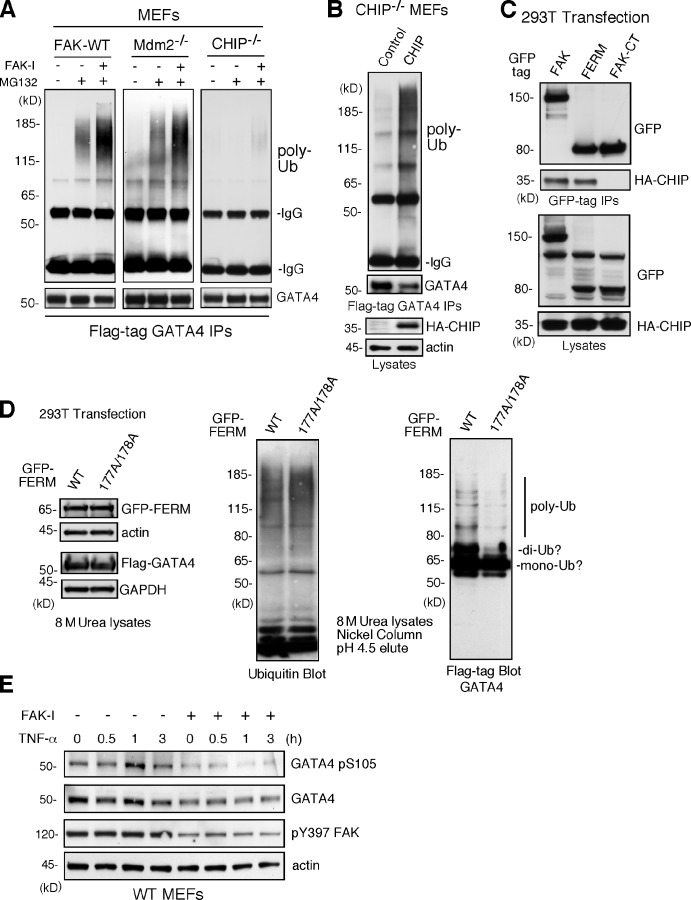
Figure 7.
FAK-enhanced GATA4 polyubiquitination is dependent on CHIP and FAK-FERM nuclear localization. (A) FAK-WT, Mdm2−/−p53−/−, and CHIP−/− MEFs were transfected with flag-tagged GATA4 and treated with MG132 (40 µM, 3 h) and FAK-I (1 µM PF271), as indicated. Flag tag immunoprecipitates (IPs) were evaluated by anti-ubiquitin (Ub) and GATA4 immunoblotting and show no ubiquitination of GATA4 in CHIP−/− MEFs. (B) CHIP−/− MEFs were transfected with HA-tagged CHIP and flag-tagged GATA4 and treated with MG132 (40 µM, 3 h) and FAK-I (1 µM PF271). Flag tag immunoprecipitates were evaluated by anti-ubiquitin and GATA4 immunoblotting and show rescue of GATA4 ubiquitination by CHIP reexpression. Lysates show HA-CHIP and actin expression. (C) 293T cells were cotransfected with GFP-tagged FAK, FAK-FERM, and FAK-CT with HA-CHIP. Coimmunoprecipitation analyses with antibodies to GFP reveal FAK-FERM and CHIP association by immunoblotting. Lysates show equal levels of GFP-FAK and HA-CHIP expression. (D) 293T cells were transfected with GFP-tagged FAK-FERM WT or FAK-FERM R177A/R178A, flag-tagged GATA4, and His-tagged ubiquitin and denatured lysates (8 M urea) purified by nickel agarose affinity binding. GFP-FERM and Flag-GATA4 (with actin and GAPDH as loading controls; left), total eluted ubiquitinated proteins (middle), and mono-, di-, and polyubiquitinated GATA4 (right), as determined by immunoblotting, are shown. (E) FAK-WT MEFs pretreated with DMSO or FAK-I (1 µM PF271, 30 min) were stimulated with 10 ng/ml TNF-α for the indicated times, and lysates were prepared for immunoblotting. Blots for activated GATA4 (pS105), total GATA4, activated FAK (pY397), and actin are shown.