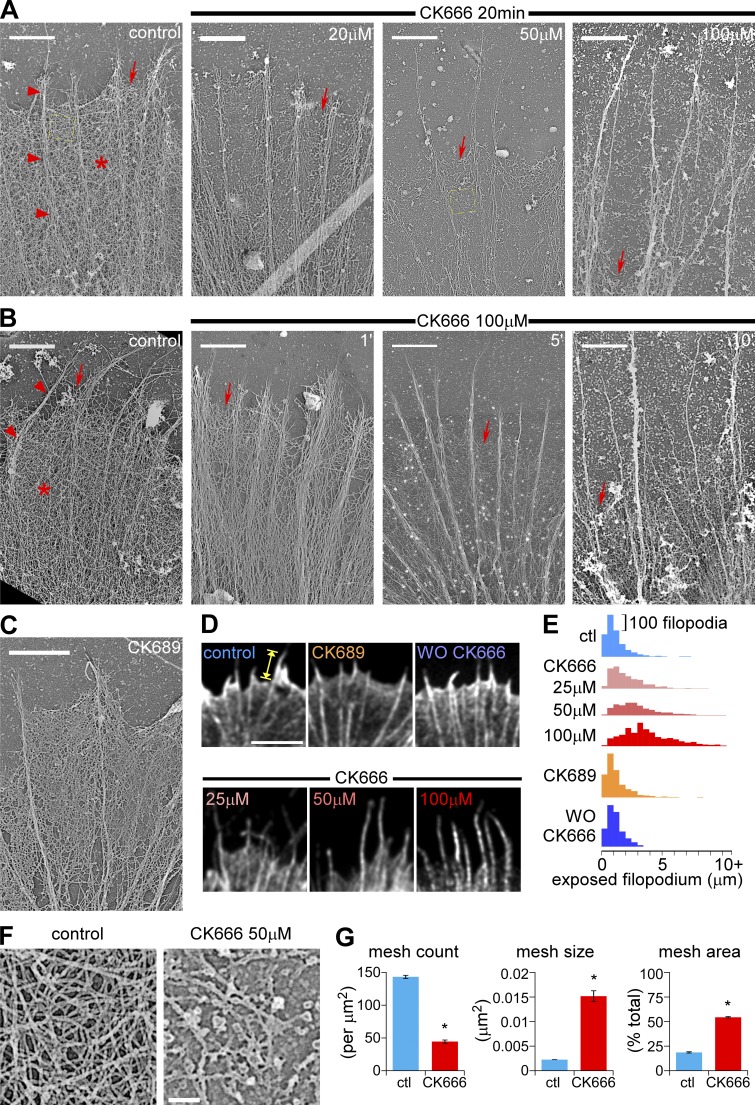
Figure 2.
Arp2/3 complex inhibition disrupts actin veil structure and leads to veil retraction in a dose- and time-dependent manner. (A) Periphery of growth cones treated with vehicle (DMSO, left) or different concentrations of CK666 for 20 min. (B) Periphery of growth cones treated with vehicle (DMSO, 20 min, left) or CK666 (100 µM) for 1, 5, or 10 min. Arrowheads, filopodial; asterisk, actin veil; arrows, edge of the veil. Yellow dashed squares are shown in F in higher magnification. (C) Periphery of a growth cone treated with CK689 (100 µM) for 20 min. For ultrastructures of the whole growth cones, see Fig. S2, E–G. (D) Leading edge of growth cones labeled with Alexa 594 phalloidin after normal fixation. Growth cones were treated with vehicle (DMSO, 20 min, top left), CK666 at different concentrations (25, 50, or 100 µM, 20 min, bottom), CK689 (100 µM, 20 min, top middle), or CK666 (100 µM, 20 min) followed by washout for 30 min (top right). See Fig. S3 A for whole growth cones. (E) Distribution of exposed filopodium lengths (yellow caliper in D) in histograms. See Fig. S3, B and C, for statistical analysis. (F) High magnification of areas marked by the yellow boxes in A showing representative veil network ultrastructure in control (left) and CK666-treated (50 µM, 20 min, right) growth cones. (G) Quantification of actin veil network parameters from 1 × 1 µm2 regions in distal P-domain similar to those in F. n, 97 regions from 8 GCs for control; 61 regions from 6 GCs for CK666. *, P < 0.01 with two-tailed unpaired t test. Bars: (A–C) 2 µm; (D) 5 µm; (F) 200 nm.