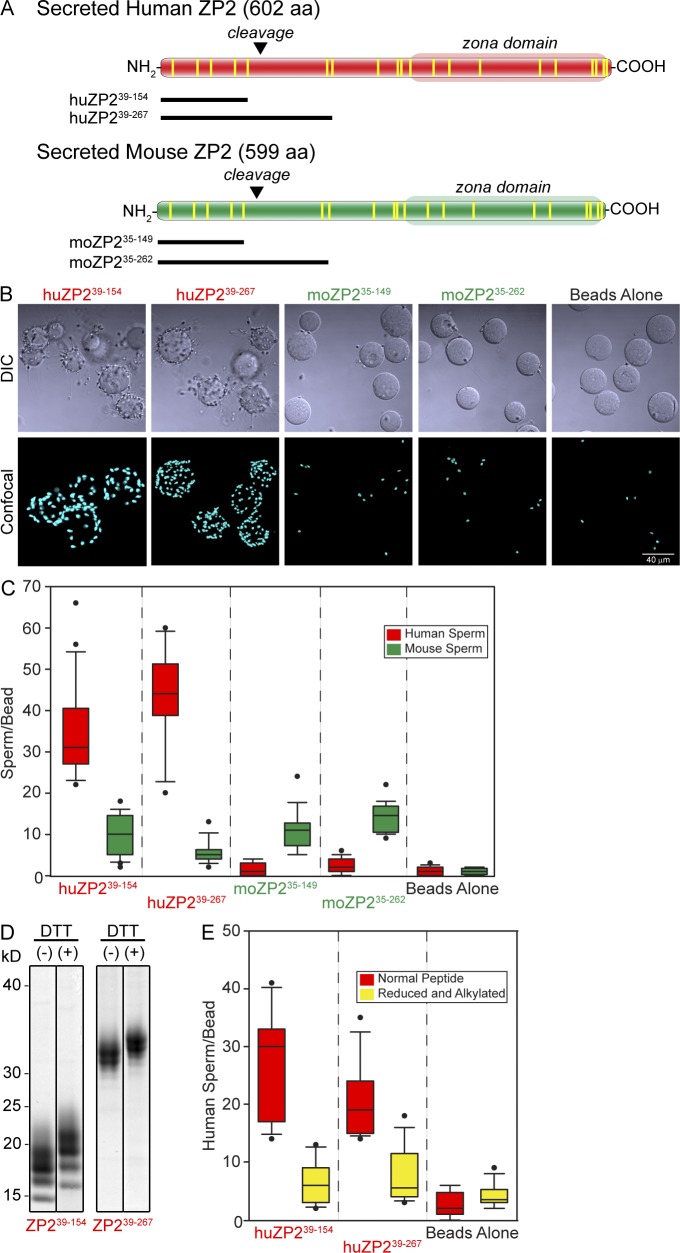
Figure 4.
Sperm binding to recombinant ZP2 peptides. (A) Schematic representation of secreted human and mouse ZP2 with zona domains at their C termini and postfertilization cleavage sites near their N termini. Vertical lines (yellow) represent conserved cysteine residues. Recombinant human and mouse N-terminal peptides that end either before or after the initial postfertilization cleavage site (inverted triangles) are indicated below each protein. (B) Capacitated human sperm binding to 6-His–tagged recombinant ZP2 peptides (huZP239–154, huZP239–267, moZP235–149, and moZP235–262) attached to IMAC Sepharose beads. Beads alone provide a negative control. DIC (top) and confocal z projection (bottom) images after staining with Hoechst. (C) Quantification of capacitated human (red) and mouse (green) sperm binding to recombinant human (huZP239–154 and huZP239–267) and mouse (moZP235–149 and moZP235–262) peptide beads. Box plots reflect the median (lines) and data points within the 10th and 90th percentiles (error bars). Boxes include the middle two quartiles, and outliers are indicated by dots. (D) Coomassie blue–stained SDS-PAGE of huZP239–154 and huZP239–267 before (−) and after (+) reduction and alkylation of the disulfide bonds. Molecular masses are indicated on the left. (E) Same as C for capacitated human sperm binding to recombinant human (huZP239–154 and huZP239–267) peptide beads before (red) and after (yellow) reduction and alkylation of peptides to disrupt disulfide bonds. Molecular masses are indicated on the left.