Table 1. Properties of the solutions used to treat developing zebrafish larvae.
| Chemical | Percentage of chemical in E3 (%) | Molarity of chemical in E3 (mM) | Osmolarity of chemical in E3 (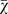 ±s) mOsm Kg−1 ±s) mOsm Kg−1
|
pH measurements; water source 1 (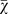 ±s) ±s) |
pH measurements; water source 2 (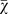 ±s) ±s) |
| Control (E3 only) | - | - | 11.63±1.19 | 5.68±0.04 | 6.17±0.09 |
| PTU + E3 | 0.003 | 0.2 | 11.75±1.58 | 5.68±0.03 | 5.94±0.02 |
| PTuracil + E3 | 0.02 | 1.175 | 12.75±0.89 | 5.75±0.02 | 5.99±0.08 |
| MM + E3 | 0.046 | 4 | 15.63±1.19 | 5.63±0.03 | 5.69±0.06 |
| DHP + E3 | 0.011 | 1 | 12.00±0.63 | 5.66±0.01 | 5.57±0.02 |
| RES + E3 | 0.0055 | 0.5 | 11.33±0.82 | 5.64±0.01 | 5.52±0.02 |
| KClO4 + E3 | 0.2 | 14.435 | 42.00±2.27 | 5.43±0.03 | 5.78±0.01 |
| KSCN + E3 | 0.0972 | 10 | 30.00±0.00 | 5.49±0.03 | 5.36±0.04 |
The osmolarity and pH of the chemicals in E3 were determined. For osmolarity, the measurements are combined from at least two different experiments because there was no difference between individual experiments (ANOVA, F(1,56) = 0.24, p-value = 0.62). See text for details. While for pH, the measurements of solutions prepared with two different sources of water were different (ANOVA, F(1,46) = 5.35, p-value = 0.025); thus each preparation is presented separately.
