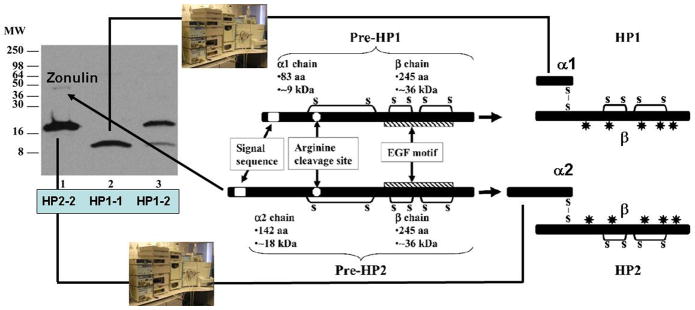
Figure 1.
Western blotting using zonulin cross-reacting anti-Zot polyclonal antibodies on CD patient sera showed three main patterns: sera showing a 18 kDa immunoreactive band and a fainter _45 kDa band (lane 1), sera showing only a 9 kDa band (lane 2), and sera showing both the 18 and 9 kDa bands (lane 3). The cartoon shows the structure of both pre-haptoglobin (HP) 1 and pre-HP2 and their mature proteins. HPs evolved from a complement-associated protein (mannose-binding lectin-associated serine protease, MASP), with their α-chain containing a complement control protein (CCP), while the β chain is related to chymotrypsin-like serine proteases (SP domain) containing an epidermal growth factor-like motif. The gene encoding the α2-chain of pre-HP2 originated in India almost 2 million years ago through a chromosomal aberration (unequal crossing over) of HP1. Pre-HPs are translated as single-chain precursor proteins. Pre-HPs may be proteolytically cleaved intracellularly into α - and β-chains that remain disulfide linked, referred to as cleaved, two-chain mature HPs.