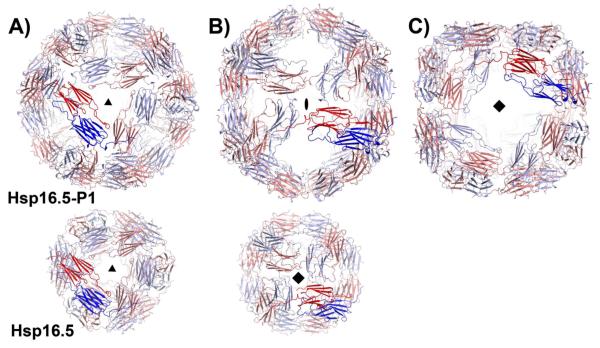
Figure 1.
Views of Hsp16.5-P1(upper row) and Hsp16.5-WT assemblies (PDBID 1SHS, lower row) along their respective symmetry axes. The two shells are scaled to highlight the expansion of the P1 variant. Hsp16.5-P1 has A) three-, B) two- and C) four-fold symmetry axes that relate dimers of the α-crystallin domain (blue and red). (B) By comparison, WT dimers of the α-crystallin domain are related by A) three- and B) four-fold symmetry.