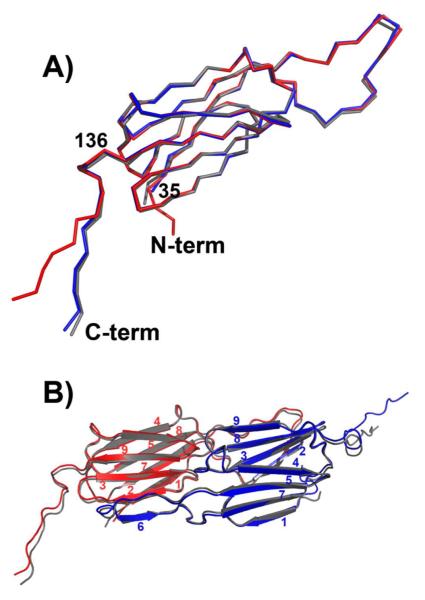
Figure 2.
A) Superposition of subunits from Hsp16.5-P1 (red and blue) and Hsp16.5-WT (gray) demonstrates that the α-crystallin domain fold is conserved and highlights the change in orientation of the C-terminal tail in one of the Hsp16.5-P1 subunits. B) Ribbon diagram of the dimer from Hsp16.5-P1 (red and blue) superimposed on the dimer from Hsp16.5-WT (gray) shows that that oligomer expansion does not perturb the interface of the dimer involving stand swapping (strand 6) and extensive networks of contacts between loops.