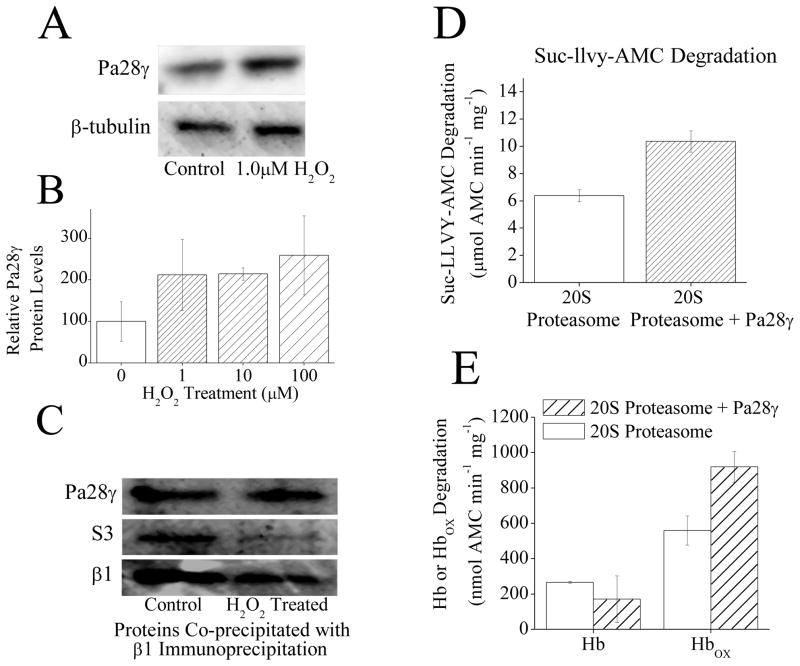
Fig. 5.
(A) H2O2 exposure causes an increase in Pa28γ protein levels. MEF cells were pretreated with ± 1μM H2O2. Then, 24 h later samples were harvested and run on Western blots which were then screened with antibodies against either Pa200 or the loading control β-tubulin. (B) H2O2 exposure causes an increase in Pa28γ protein levels. MEF cells were prepared as in panel A in triplicate. Sample band intensity was then measured and adjusted based on β-tubulin band intensity. Values are means ± SE, where n = 3. (C) Following H2O2 treatment there is no change in Pa28γ association with 20S proteasome. MEF cells were exposed to 1mM H2O2 for 1 h, after which immunoprecipitation of anti-α5 was performed. Samples were run on Western blots and screened for co-immunoprecipitation of anti-Pa28γ, or anti-S3 (a subunit of the 19S regulator) as a measure of 26S proteasome disassembly. anti-α5 (not shown) was used as a loading control to confirm similar levels of 20S proteasome. The β1 blot was taken from a separate set of samples run under the same conditions. (D) Pa28γ slightly increases the capacity of 20S proteasome to degrade the peptide substrate Suc-LLVY-AMC. Purified 20S proteasome was incubated with a 4 fold molar excess of Pa28γ for 30 minutes. Suc-LLVY-AMC was then added to samples and proteolytic capacity measured. Values are means ± SE, where n = 3. (E) Pa28γ increases the capacity of 20S proteasome to degrade oxidized hemoglobin but not native hemoglobin. Purified 20S proteasome was incubated with a 4 fold molar excess of Pa28γ for 30 minutes. Hb-AMC or HbOX-AMC was then added to samples and proteolytic capacity measured. Values are means ± SE, where n = 8.