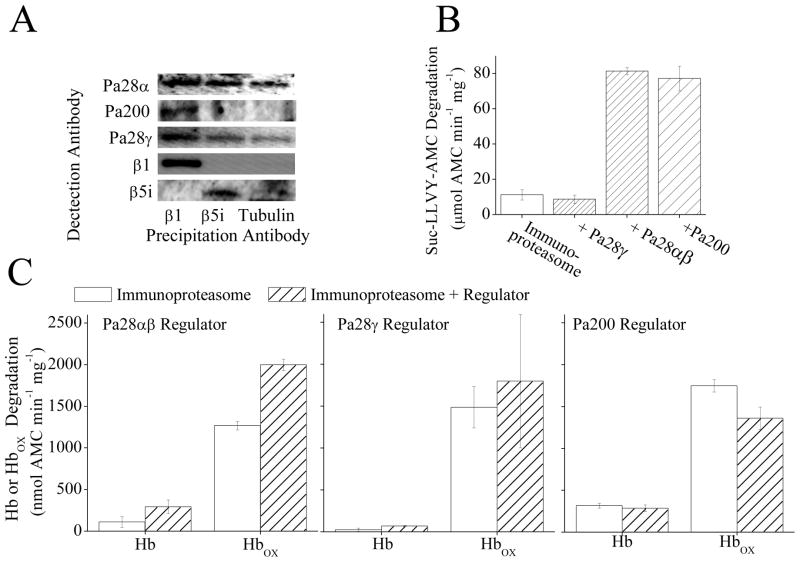
Fig. 6.
(A) Immunoprecipitation of MEF cell lysate with an antibody against the 20S proteasome subunit β1 causes co-precipitation of Pa28αβ, Pa200, and Pa28γ. However immunoprecipitation with an antibody against the immunoproteasome subunit β5i causes weak co-precipitation of Pa28αβ, and Pa28γ but not Pa200. Immunoprecipitation of MEF cells was performed using anti-β1, anti-β5i, or anti-β-tubulin (as a control for non-specific binding) antibodies. The Immunoprecipitate was analyzed by Western blotting and samples were screened for co-immunoprecipitation of anti-Pa28α, anti-Pa200, anti-Pa28γ, anti-β1, or anti-β5i. (B) Pa28αβ and Pa200 but not Pa28γ increase the capacity of immunoproteasome to degrade the peptide substrate Suc-LLVY-AMC. Immunoproteasome was incubated with a 4 fold molar excess of either Pa28γ Pa28αβ or Pa200 for 30 minutes. Suc-LLVY-AMC was then added to samples and proteolytic capacity measured. Values are means ± SE, where n = 3. (C) Binding of Pa28αβ (left panel) but not Pa28γ (center panel) or Pa200 (right panel) significantly increases the selective degradation of oxidized hemoglobin by immunoproteasome. Samples were prepared as in A, then AMC labeled hemoglobin or AMC labeled oxidized hemoglobin were added to samples as substrates. Values are means ± SE, where n = 8.