Fig. 8.
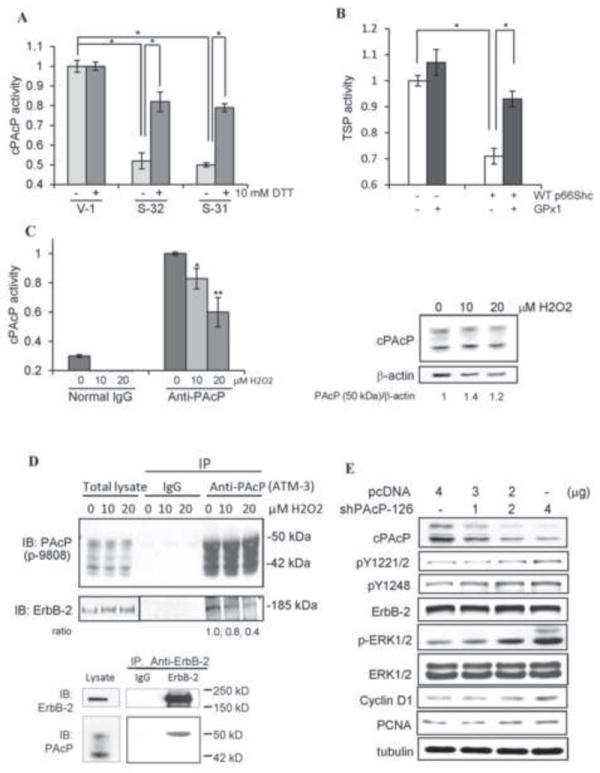
Oxidation-induced inactivation of cPAcP in p66Shc cDNA-transfected PCa cells. (A) The total cell lysate proteins from S-31, S-32 and V-1 cells were prepared for immunoprecipitation of cPAcP. The immunocomplexes was then incubated with or without 10 mM DTT for 10 min at room temperature. The cPAcP-specific activity in the immunocomplexes was quantified. Similar results were obtained from at least three sets of independent experiments. The data shown is one set of representative results. (*p<0.05). (B) LNCaP C-33 cells were transiently co-transfected with WT p66Shc (WT) cDNA plus glutathione peroxidase 1 (GPx1) cDNA. Control cells were transfected with p66Shc cDNA, GPx1 cDNA or the vector alone. After 48 h, cells were harvested for analyzing TSP activity. Similar results were obtained from two sets of independent experiments. (*p<0.05). (C) LNCaP C-33 cells were seeded in regular medium and then maintained in SR medium for 2 days. After being treated with 0, 10 and 20 μM H2O2 for 24 h, total cell lysate proteins were used to immunoprecipitate cPAcP by anti-PAcP Ab. Normal IgG was used as a control for immunoprecipitation. The AcP activity in the immunocomplex was quantified (left panel, n=2×3; *p<0.05; **p<0.01). The total cell lysate proteins were analyzed for cPAcP protein levels. β-actin protein level was used as a loading control. (D) Interaction of cPAcP and ErbB-2 in LNCaP C-33 cells. (Upper panel) After attachments for 3 days in regular medium, LNCaP C-33 cells were maintained in a SR medium for 48 h and exposed to 0, 10 and 20 μM H2O2. Cells were then harvested, lysed and immunoprecipitated by anti-PAcP Ab (#ATM-3) or normal IgG as a control. The immunoprecipitate was used for western blotting with anti-PAcP Ab (Sigma #P-9808) and anti-ErbB-2 Ab (C-18). Similar results were obtained from three sets of independent experiments. (Lower panel) ErbB-2 was immunoprecipitated from steroid-starved LNCaP C-33 cell lysate proteins using anti-ErbB-2 mouse IgG (9G6 Ab) coupled to protein G-Sepharose beads. The immunoprecipitate complexes were analyzed by western blotting with anti-ErbB-2 rabbit IgG (C-18 Ab) and anti-PAcP mouse Ab (Sigma, USA). Only the 50 kD form of PAcP was seen to be co-immunoprecipitated with ErbB-2 even after prolonged exposure. The data is the representative of five sets of independent experiments. (E) Effect of transient knockdown of cPAcP by shRNA in LNCaP C-33 cells on tyrosine phosphorylation of ErbB-2. LNCaP C-33 cells were transiently transfected with different amounts of PAcP shRNA-126 plasmids. Control cells were transfected with vector containing scrambled oligonucleotides (pcDNA). Total lysate proteins were analyzed for cPAcP protein levels, the site-specific ErbB-2 phosphorylation, ERK phosphorylation, cyclin D1 and PCNA protein levels. Tubulin was used as a loading control.
