Abstract
Prostate cancer is a slowly developing but very common cancer in males that may be amenable to preventive strategies that are not toxic. Chinese red yeast rice (RYR), a food herb made by fermenting Monascus purpureus Went yeast on white rice, contains a mixture of eight different monacolins that inhibit cholesterogenesis in addition to red pigments with antioxidant properties. Monacolin K is identical to lovastatin (LV), but lovastatin unlike RYR can be used in individuals intolerant to statins due to muscle pain. Both LV and RYR inhibit de novo cholesterogenesis, which is critical to the growth of tumor cells. Long-term use of statin drugs has been associated with a reduced risk of prostate cancer. We have previously shown that RYR inhibited androgen-dependent and AR-overexpressing androgen-independent prostate cancer cell proliferation in vitro. The present study was designed to determine whether RYR and LV inhibit prostate tumor growth in SCID mice. RYR significantly reduced tumor volumes of androgen-dependent and androgen-independent prostate xenograft tumors compared to animals receiving vehicle alone (P<0.05). Inhibition by RYR was greater than that observed with LV at the dose found in RYR demonstrating that other compounds in RYR contributed to the antiproliferative effect. There was a significant correlation of tumor volume to serum cholesterol (P<0.001). RYR decreased gene expression of androgen synthesizing enzymes (HSD3B2, AKR1C3 and SRD5A1) in both type of tumors (P<0.05). Clinical studies of RYR for prostate cancer prevention in the increasing population of men undergoing active surveillance should be considered.
Keywords: Chinese red yeast rice, cholesterogenesis, lovastatin, prostate cancer, xenograft
Introduction
Prostate cancer (PCa) is the second most common cause of cancer death in men in the United States today (1). It is estimated that 217,730 new cases occurred and 32,050 men will die of prostate cancer in 2010 (1). Early stage prostate cancer is androgen-dependent and can be effectively treated by androgen ablation therapy, radiation and/or surgery (2-7). However prostate tumors relapse and advance to an androgen-independent state where they progress in the absence of circulating testosterone, leading to metastasis and death (2-7). Reducing the rate of emergence of androgen-independent cells in late stages of advanced prostate cancer is critical to reducing overall mortality from prostate cancer.
Red Yeast Rice (RYR) is a traditional food spice consumed throughout Asia (8-11) and its food and medicinal value is believed to date back more than a thousand years. RYR contains a family of monacolins, one of which is monacolin K, which is identical to lovastatin (LV), and has the ability to inhibit cholesterol synthesis and lower plasma cholesterol levels (12, 13). Our group demonstrated that the administration of a dose of 2400 mg/day RYR, containing 0.4% monacolins by weight, to hypercholesterolemic participants resulted in significant reduction of total cholesterol and LDL cholesterol with approximate bioequivalence to 20 mg of lovastatin, suggesting that other substances in RYR had biological activity (14).
A recent case-control study (15) reported that hypercholesterolemia was associated with a 50 % increase in the risk of prostate cancer. In clinical studies, statin drug use was protective against prostate cancer (16-24) although the mechanisms of this effect have not been established. Recently, we demonstrated that RYR could inhibit in vitro proliferation of both LNCaP human prostate cancer cells and LNCaP-AR cells which are androgen-independent and overexpress the androgen receptor (25).
Androgen-independent cells synthesize testosterone intracellularly via several enzymes which support tumor growth in the absence of circulating androgens (26-30). 3β-hydroxysteroid dehydrogenase type 2 (HSD3B2) catalyzes the conversion of dehydroepi-androsterone (DHEA) to androstenedione (26, 27). In addition, aldo-keto reductase family 1, member C3 (AKR1C3) converts androstenedione to testosterone and increased amounts of AKR1C3 have been demonstrated in prostate cancer cells (28). Testosterone is converted to dihydrotestosterone (DHT) by 5α-reductase (SRD5A1) (29). Since DHT has a higher affinity for AR than testosterone, it has been proposed that DHT is critical to prostate cancer development (27). Inhibitors of SRD5A1, such as finasteride, reduce prostate size and have been shown to reduce the development of prostate cancers by 25 percent but to increase the numbers of advanced cancers found (31, 32). Therefore, androgen synthesis enzymes may be critical for the development of androgen-independent prostate cancer.
The present study examined the effects of RYR on the growth of androgen-dependent LNCaP and androgen-independent LNCaP-AR human prostate cancer xenografts in severe combined (SCID) mice as well as the relationship of circulating cholesterol to tumor growth and the effects of treatment with RYR on gene expression of androgen-synthesizing enzymes.
Materials and Methods
SCID animals and diets
Sixty male SCID mice, aged 5 weeks, were purchased from Taconic Farms Inc. (Hudson, NY) and housed 5 mice per cage in a pathogen-free environment. After acclimation, all mice were implanted in the shoulder with androgen-dependent LNCaP or androgen-independent LNCaP-AR human prostate cancer cells (8 × 106) subcutaneously. LNCaP cells were purchased from the American Type Culture Collection (ATCC, Rockville, MD) and lower passages (4-6 passages) of cells were used. LNCaP-AR cells were provided from Dr. Charles Sawyers' lab (University of California, Los Angeles, CA) three months prior to the experiments. The LNCaP and LNCaP-AR cells were authenticated by Pathogen PCR Testing in Division of Laboratory Animal Medicine (DLAM Lab, University of California, Los Angeles, CA) right before the xenograft study.
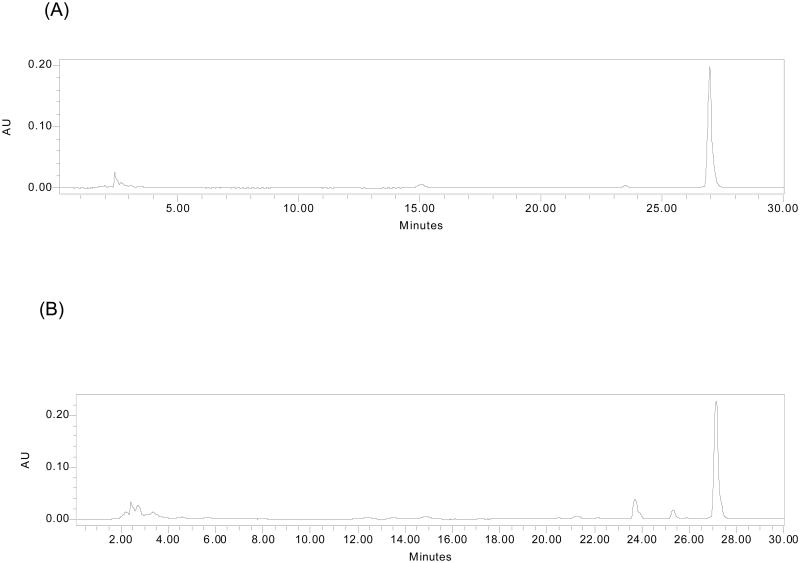
Mice were provided with control, lovastatin or RYR diet. The control diet was modified AIN 93G diet (Dyets, Bethehem, PA) with 20% fat (20% soybean oil). The RYR diet contained 5% of RYR powder (Botanica Bioscience, Ojai, CA) with the modified AIN93G diet. For lovastatin diet, lovastatin (Mylan pHarmaceuticals Inc., Morgantown, WV) was added to the control diet in an amount equivalent to that in 5% RYR diet. The amounts present in the lovastatin diet and 5% RYR diet were found to be nearly identical after determination by HPLC (Figure 1).
Figure 1.
HPLC chromatograms of monacolin K in lovastatin diet (A) and 5% RYR diet (B). The amounts of lovastatin were very similar in both diets.
Animal weight, food intake and tumor volume were measured weekly. The tumor volume was calculated using the formula: length × width × height × 0.5236 (33). At sacrifice, primary tumors were excised and blood was collected through cardiac puncture. The animal protocol was approved by Animal Care Committee of the University of California, Los Angeles.
Serum cholesterol and PSA
Serum cholesterol concentrations were determined by cholesterol enzymatic methods using a cholesterol standard (StanBio, Boerne, TX). Serum PSA was measured with a PSA ELISA kit (Diagnostic Systems Laboratories, Webster, TX) according to the manufacturer's protocol.
In situ cell proliferation and apoptosis
Paraformaldehyde-fixed and paraffin-embedded tumor tissues were used to determine in situ cell proliferation and apoptosis analysis. Proliferating cells were detected using monoclonal Ki-67 antibody (BD Biosciences, San Diego, CA) (34, 35). Total number of cells and stained proliferating cells were counted in 2 sub-squares in a 4 × 4 grid in 10 microscopic areas. Data are expressed as proliferation index (%) which was calculated by the equation [(# of proliferating cells in one grid/total number of cells in one grid)* 100)].
Apoptosis assay was based on terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-biotin nick end labeling (Millipore, Billerica, MA) (34). Apoptotic cells were counted in 15 microscopic fields and data are expressed in number of apoptotic cells/field.
RNA extraction and reverse transcription
Total RNA was extracted using TRIZOL reagent (Invitrogen, Carlsbad, CA) and quantified by measuring the absorbance at 260 nm with a Gene Quant Spectrophotometer (Amersham-Pharmacia Biotech, Piscataway, NJ). Reverse transcription was performed on 3 μg of RNA by using oligo(dT)12–18 primers (Invitrogen, Carlsbad, CA) with SuperScript III reverse transcriptase (Invitrogen) according to the manufacturer's instructions.
Quantitative real time PCR
Gene expressions were determined using Taqman Universal PCR master mix and primers (Applied Biosystems, Foster City, CA) by quantitative real time polymerase chain reaction (PCR) using the ABI 7900 HT Sequence Detector (Applied Biosystems) (36). The transcription levels of target genes were normalized to r18S expression. Every set of reverse transcription reactions contains a minus reverse transcription negative control to confirm that no contamination or anomaly has occurred.
Statistics
Data were analyzed by one-way ANOVA followed by Student-Newman-Keuls (SNK) multiple comparison with GraphPad PRISM 3.0 (GraphPad Software, San Diego, CA) in separate androgen-dependent and -independent SCID sets. The comparison between LNCaP and LNCaP-AR were done using t-test. The relationship between tumor volume and serum cholesterol levels was analyzed using correlation and a linear regression model.
Results
SCID Tumor volume
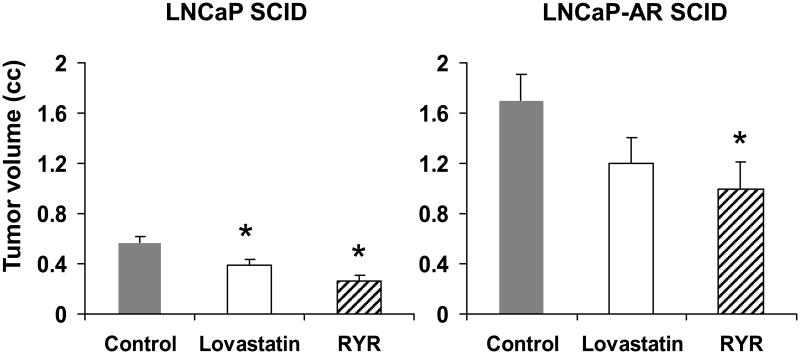
RYR significantly reduced androgen-dependent and androgen-independent tumor volumes compared to control by 54% and 41%, respectively (P<0.05) (Figure 2). Lovastatin also decreased tumor volume by 32% (P<0.05) but only in androgen-dependent SCID animals, and to a lesser degree than those receiving RYR (Figure 2).
Figure 2.
RYR effects on SCID tumor volume. RYR-fed mice significantly reduced androgen-dependent and androgen-independent tumor compared to control diet-fed mice. Values are Mean ± SE. *: Significantly different from control at P<0.05.
In situ cell proliferation and apoptosis
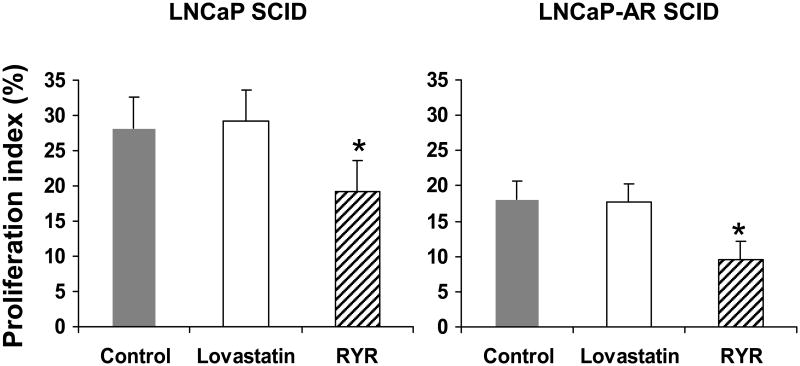
Proliferation index was lower in the RYR group in both androgen-dependent and – independent SCID tumors by 32% and 47% (P<0.05) (Figure 3). There was no significant effect of RYR or lovastatin on apoptosis (data not shown).
Figure 3.
RYR effects on in situ cell proliferation. Proliferation index was lower in RYR group in both androgen-dependent and -independent SCID tumors Values are Mean ± SE. *: Significantly different from control at P<0.05.
Serum PSA
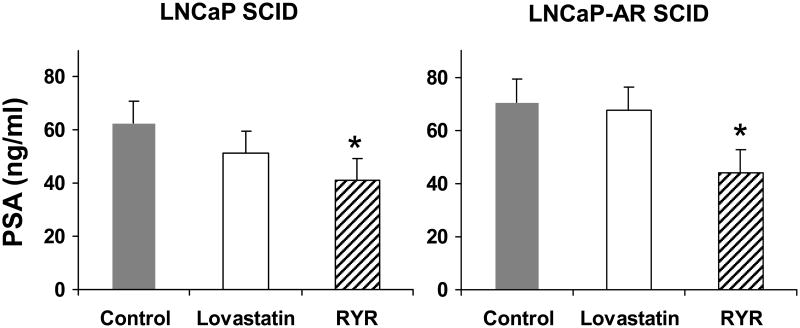
PSA is a risk marker of prostate cancer and is often elevated in the presence of prostate cancer. RYR decreased serum PSA levels compared to control in both LNCaP and LNCaP-AR injected SCID animals (P<0.05) (Figure 4).
Figure 4.
RYR effects on PSA levels. RYR administration reduced serum PSA levels compared to control in both types of SCID mice. Values are Mean ± SE. *: Significantly different from control at P<0.05.
Serum cholesterol and HMGCR gene expression
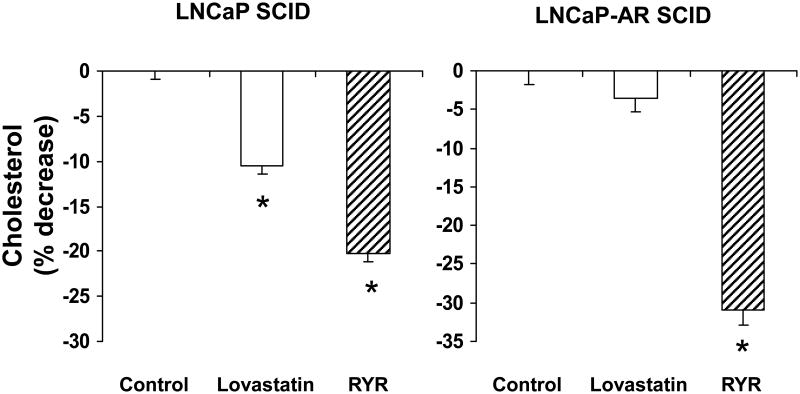
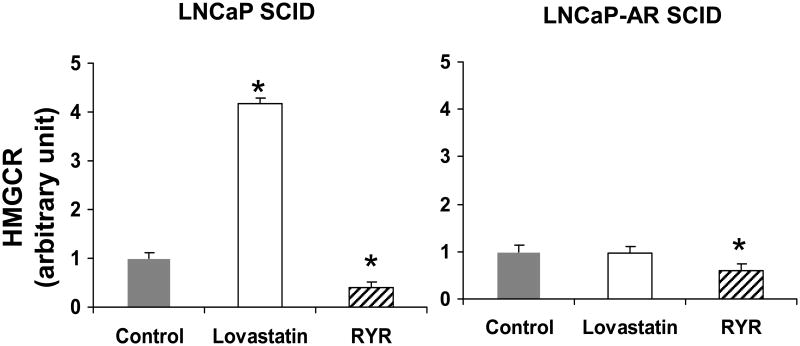
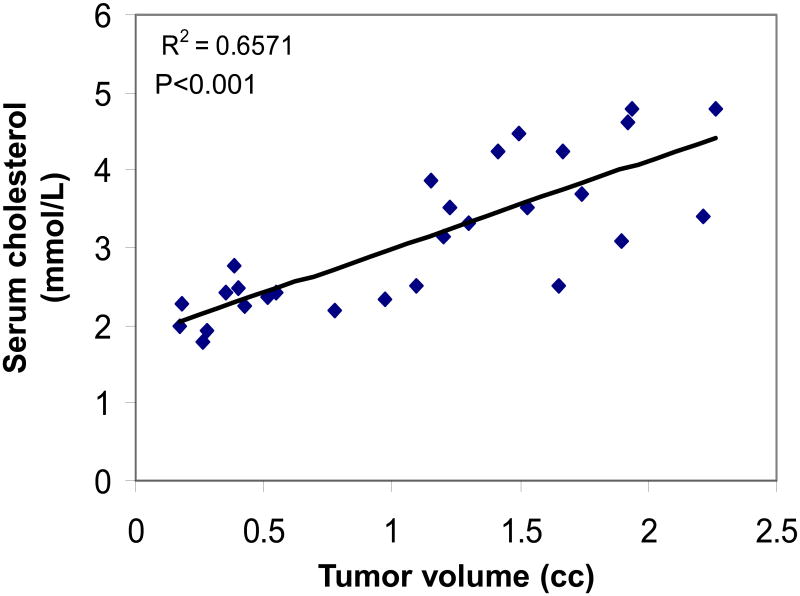
RYR decreased serum cholesterol levels by 20% in animals with androgen-dependent tumors, and 30% in animals with androgen-independent tumors (P<0.05) (Figure 5). In animals treated with the lovastatin diet, serum cholesterol levels declined by 10% in animals with androgen-dependent tumors, but not in those with androgen-independent tumors (P<0.05) (Figure 5). The RYR diet downregulated HMGCR gene expression by 60% in animals with androgen-dependent tumors and 40% in animals with androgen-independent tumors. (P<0.05) (Figure 6). In contrast, lovastatin-treated animals had a fourfold increase in HMGCR gene expression compared to animals receiving the control diet (Figure 6). There was a strong positive correlation between tumor volume and serum cholesterol levels (R2=0.6571, P<0.001) (Figure 7).
Figure 5.
RYR effects on cholesterol levels. RYR reduced serum cholesterol levels more than 15% of control in androgen-dependent and –independent SCID mice. Values are Mean ± SE. *: Significantly different from control at P<0.05.
Figure 6.
RYR effects on HMGCR gene expression. RYR downregulated HMGCR gene expression in both types of xenografted tumors. In contrast, lovastatin drug increased HMGCR gene expression in LNCaP xenograft animal. Values are Mean ± SE. *: Significantly different from control at P<0.05.
Figure 7.
Correlation between tumor volume and serum cholesterol levels. As tumors were getting bigger, serum cholesterol increased or vise versa (R2=0.6571, P<0.001). All data were combined.
Gene expression of androgen synthesizing enzymes and androgen receptor (AR)
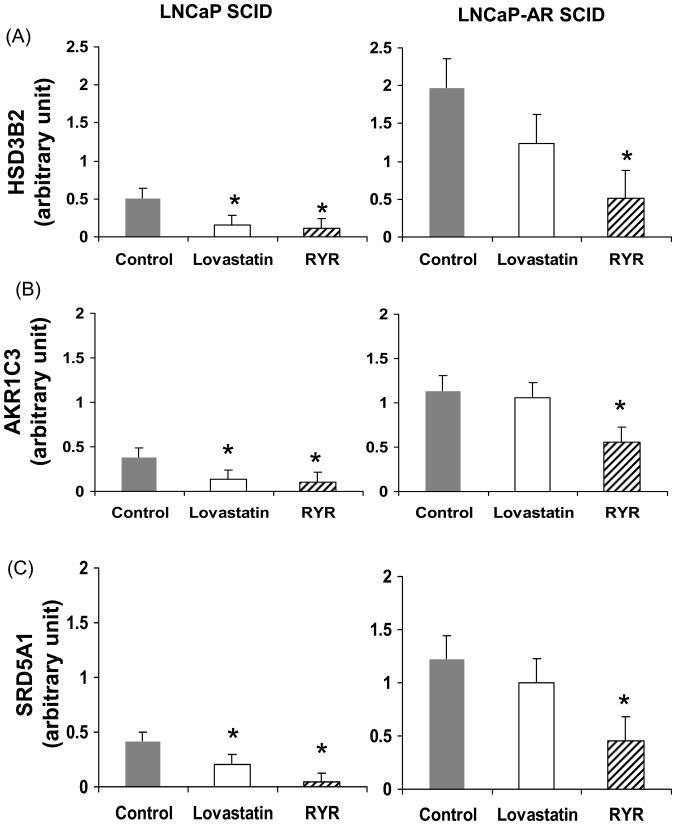
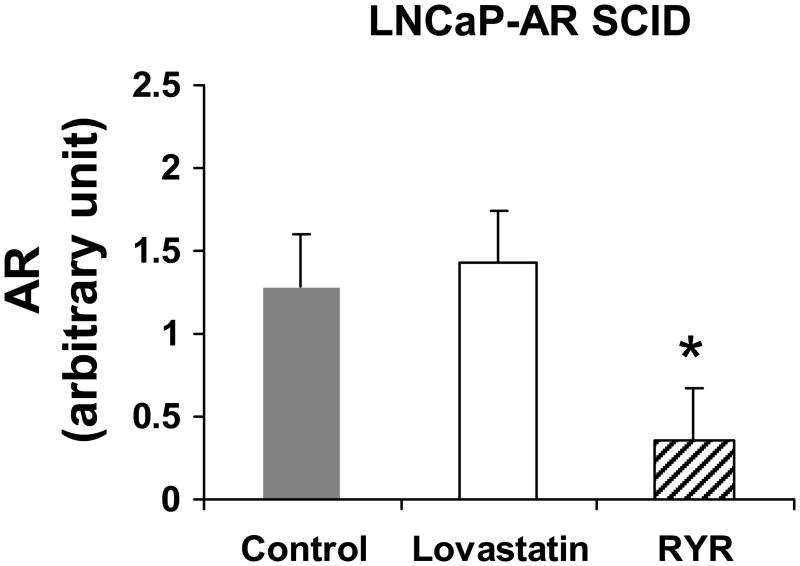
In RYR-fed animals, HSD3B2, AKR1C3 and SRD5A1 gene expression was downregulated more than threefold in LNCaP tumors, and more than twofold in LNCaP-AR tumors (P<0.05) (Figure 8A, 8B and 8C). The RYR diet also decreased AR expression more than twofold in androgen-independent SCID tumors (P<0.05) (Figure 9). The transcription levels of HSD3B2, AKR1C3 and SRD5A1 and AR were higher in androgen-independent tumors than androgen-dependent tumors (P<0.05) (Figures 8 and 9).
Figure 8.
RYR effects on gene expression involved in androgen synthesis. RYR-fed animals downregulated HSD3B2 (A), AKR1C3 (B) and SRD5A1 (C) gene expression by more than three and two folds in LNCaP and LNCaP-AR SCID tumors. Values are Mean ± SE. *: Significantly different from control at P<0.05.
Figure 9.
RYR effects on gene expression of androgen receptor (AR). RYR diet also decreased AR expression by more than 2 folds in androgen-independent SCID tumors. Values are Mean ± SE. *: Significantly different from control at P<0.05.
Discussion
Androgens are critical to prostate cancer development as well as to normal development, proliferation, and differentiation of prostate epithelial cells (2, 3). Androgen deprivation therapy is used after failure of primary prostate cancer, but the emergence of androgen-independent prostate cancer often occurs. While cholesterogenesis is necessary for testosterone synthesis (30), there is no convincing evidence in humans that reducing cholesterol biosynthesis affects circulating testosterone levels (37). Cholesterol-rich membranes, generally referred to as “lipid rafts,” exhibit a liquid-ordered structure and are insoluble in non-ionic detergents. Lipid rafts sequester and exclude certain types of signaling proteins and are thought to act as membrane platforms for signal transduction. The depletion of cholesterol from the lipid raft could inhibit prostate tumor xenograft growth (38). On the other hand, cholesterol is a required intermediate for tumor cell growth, and reduction of circulating cholesterol levels may influence the risk of progression and biology of prostate cancer (39, 40). While we and others (38) have seen a correlation between xenograft size and circulating cholesterol levels, further research is needed to clarify which of these mechanisms are most likely involved in tumor growth inhibition.
The mechanisms by which prostatic tissue maintains tissue androgens may include metabolism of adrenal androgens or de novo synthesis from cholesterol (41, 42). The current study verified that higher cholesterol levels were strongly correlated with larger prostate tumors. RYR downregulated enzymes involved in androgen synthesis (HSD3B2, AKR1C3 and SRD5A1) and reduced cholesterol levels. RYR may decrease androgen synthesis by reducing the precursor (i.e. cholesterol) of androgen via the inhibition of de novo cholesterogenesis and downregulation of androgen synthesizing enzyme genes. In patients undergoing androgen deprivation therapy to treat prostate cancer, RYR could influence disease progression via effects on residual androgen production.
Androgens signaling occurs via intracellular AR (2, 3, 43). The AR has been implicated in the development and progression of recurrent prostate cancer and its expression is frequently upregulated in androgen-independent prostate cancer (44-46). This enhances the response to circulating androgens and those synthesized in the prostate cancer cell. This upregulation of the AR in androgen-independent tumors was consistent with our findings in which RYR downregulated AR transcription levels in the animals with androgen-independent tumors. Therefore, RYR may be particularly helpful in the subgroup of patients with androgen-independent prostate cancer and AR upregulation.
De novo cholesterogenesis may be a key target for the prevention of the emergence of prostate cancer. Much convincing evidence indicates that tumors undergo deregulated cholesterogenesis mainly at the critical rate-controlling juncture (i.e., the reaction catalyzed by HMGCR). The mevalonate component of the cholesterol biosynthesis pathway plays a key role in controlling cell proliferation by generating prenyl intermediates, particularly farnesyl and geranyl-geranyl moieties (47-49). These isoprenoids covalently modify, and thus modulate, the biological activity of signal transducing proteins, such as G-protein signaling. Further studies are needed to determine the effects of RYR on isoprenoid metabolism and related signaling.
We have previously shown the anticancer properties of RYR in human prostate cancer cell lines (25). The in vitro effects demonstrated that RYR exhibited stronger inhibition of tumor cell growth compared to LV treatment in human androgen-dependent and –independent prostate cancer cells (25). The results are extended in the current in vivo xenograft study. It is also interesting that LV administration enhanced HMGCR gene expression in androgen-dependent tumors but RYR downregulated the transcription levels in both types of tumor. The advantage of using RYR over LV, which is a drug, is that RYR reduced tumor volume, PSA levels and cholesterol levels without elevation of gene expression related to HMGCR, androgen synthesis and inflammation. Moreover, RYR showed anti-cancer effects in both androgen-dependent and – independent prostate tumors while effects of LV were observed mainly in androgen-dependent SCID prostate tumors.
The amount of Red Yeast Rice typically used in clinical trials is 1200-2400 mg/day of red yeast rice containing approximately 10 mg total monacolins of which half is monacolin K. This raises a question about function of the other monacolins and non-monacolin compounds in the products, as the monacolin K content is lower than the low end of what is usually considered effective for lovastatin (10-80 mg/day). A meta-analysis published in 2006 cited 93 published, controlled clinical trials − 91 published in Chinese. Compared to placebo results, total cholesterol decreased by 35 mg/dl, LDL-cholesterol by 28 mg/dl, triglycerides by 35 mg/dl, and HDL-cholesterol increased by 6 mg/dl (50). Of the clinical trials reviewed in the meta-analysis, the only study conducted in the United States was a randomized, placebocontrolled, double-blind study of subjects with primary hyperlipidemia (LDL>160 mg/dL). At the end of 12 weeks it reported changes compared with placebo of -35, -33, -13 and 0 mg/dL for total cholesterol, LDL, triglycerides, and HDL respectively (14). While RYR has demonstrated inhibition of androgen-dependent and –independent prostate tumors in our studies, it is also likely to be better tolerated than statin drugs. In a recent study (51), patients experiencing myalgias, gastrointestinal intolerance, or elevated liver function tests with lipid-lowering drugs were able to tolerate RYR without any side effects. Based on our basic research and clinical observations of RYR in lipid lowering trials, it is our view that clinical studies of RYR for prostate cancer prevention in men undergoing active surveillance should be considered.
Acknowledgments
We thank Dr. Simin Liu for allowing us to use his real time PCR equipment. This study was funded by UCLA/NCI Clinical Nutrition Research Unit Grant No. CA 42710. This work was also funded by W81XWH-07-1-0158 grant from Department of Defense (PI: MYH).
Abbreviations
- AKR1C3
aldo-keto reductase family 1, member C3
- AR
androgen receptor
- DHT
dihydrotestosterone
- ELISA
enzyme-linked immunosorbent assay
- HMGCR
3-hydroxy-3-methyl-glutaryl CoA reductase
- HPLC
high performance liquid chromatography
- HSD3B2
3β-hydroxysteroid dehydrogenase type 2
- LV
lovastatin
- MK
monacolin K
- MV
mevalonate
- PCa
prostate cancer
- PCR
polymerase chain reaction
- PSA
prostate specific antigen
- RYR
Chinese red yeast rice
- SCID
severe combined immunodeficiency
- SRD5A1
steroid 5α reductase type 1
References
- 1.American Cancer Society. Cancer facts and figures. 2010 www.cancer.org.
- 2.Attard G, Sarker D, Reid A, Molife R, Parker C, de Bono JS. Improving the outcome of patients with castration-resistant prostate cancer through rational drug development. Br J Cancer. 2006;95:767–74. doi: 10.1038/sj.bjc.6603223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–9. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 4.Kokontis JM, Hay N, Liao S. Progression of LNCaP prostate tumor cells during androgen deprivation: hormone-independent growth, repression of proliferation by androgen, and role for p27Kip1 in androgen-induced cell cycle arrest. Mol Endocrinol. 1998;12:941–53. doi: 10.1210/mend.12.7.0136. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L, Johnson M, Le KH, et al. Interrogating androgen receptor function in recurrent prostate cancer. Cancer Res. 2003;63:4552–60. [PubMed] [Google Scholar]
- 6.Jariwala U, Prescott J, Jia L, et al. Identification of novel androgen receptor target genes in prostate cancer. Mol Cancer. 2007;6:39–53. doi: 10.1186/1476-4598-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim D, Gregory CW, French FS, Smith GJ, Mohler JL. Androgen receptor expression and cellular proliferation during transition from androgen-dependent to recurrent growth after castration in the CWR22 prostate cancer xenograft. Am J Pathol. 2002;160:219–26. doi: 10.1016/S0002-9440(10)64365-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma J, Li Y, Ye Q, et al. Constituents of red yeast rice, a traditional Chinese food and medicine. J Agric Food Chem. 2000;48:5220–5. doi: 10.1021/jf000338c. [DOI] [PubMed] [Google Scholar]
- 9.Wang HL, Fang SL. Indigenous fermented foods of non-western origin. In: Hesseltine CW, Wang HL, editors. Mcologia Memoirs. 1986. pp. 317–44. [Google Scholar]
- 10.Simg RH. Tien-Kung K'ai-Wu Chinese Technology in the Seventeenth Century. Pennsylvania State University Press; 1996. pp. 292–4. [Google Scholar]
- 11.Stuart MD. Chinese material medica: Vegetable kindom. Taipai, Republic of China: Southern materials Center; 1979. [Google Scholar]
- 12.Plasma clearance of lovastatin versus Chinese red yeast rice in healthy volunteers. J Altern Complement Med. 2005;11:1031–8. doi: 10.1089/acm.2005.11.1031. [DOI] [PubMed] [Google Scholar]
- 13.Heber D, Lembertas A, Lu QY, Bowerman S, Go VLW. An analysis of nice proprietary Chinese Red Yeast Rice dietary supplements: Implications of variability in chemical profile and contents. J Altern Complement Med. 2001;7:133–9. doi: 10.1089/107555301750164181. [DOI] [PubMed] [Google Scholar]
- 14.Heber D, Yip I, Ashley JM, Elashoff DA, Elashoff RM, Go VL. Cholesterol-lowering effects of a proprietary Chinese red-yeast-rice dietary supplement. Am J Clin Nutr. 1999;69:231–6. doi: 10.1093/ajcn/69.2.231. [DOI] [PubMed] [Google Scholar]
- 15.Bravi F, Scotti L, Bosetti C, et al. Self-reported history of hypercholesterolaemia and gallstones and the risk of prostate cancer. Ann Oncol. 2006;17:1014–7. doi: 10.1093/annonc/mdl080. [DOI] [PubMed] [Google Scholar]
- 16.Cyrus-David MS, Weinberg A, Thompson T, Kadmon D. The effect of statins on serum prostate specific antigen levels in a cohort of airline pilots: a preliminary report. J Urol. 2005;173:1923–5. doi: 10.1097/01.ju.0000158044.94188.88. [DOI] [PubMed] [Google Scholar]
- 17.Shannon J, Tewoderos S, Garzotto M, et al. Statins and prostate cancer risk: a case-control study. Am J Epidemiol. 2005;162:318–25. doi: 10.1093/aje/kwi203. [DOI] [PubMed] [Google Scholar]
- 18.Moyad MA, Merrick GS, Butler WM, et al. Statins, especially atorvastatin, may favorably influence clinical presentation and biochemical progression-free survival after brachytherapy for clinically localized prostate cancer. Urology. 2005;66:1150–4. doi: 10.1016/j.urology.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 19.Gutt R, Tonlaar N, Kunnavakkam R, Karrison T, Weichselbaum RR, Liauw SL. Statin use and risk of prostate cancer recurrence in men treated with radiation therapy. J Clin Oncol. 2010;28:2653–9. doi: 10.1200/JCO.2009.27.3003. [DOI] [PubMed] [Google Scholar]
- 20.Katz MS, Carroll PR, Cowan JE, Chan JM, D'Amico AV. Association of statin and nonsteroidal anti-inflammatory drug use with prostate cancer outcomes: results from CaPSURE. BJU Int. 2010 doi: 10.1111/j.1464-410X.2010.09232.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 21.Demierre MF, Higgins PD, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer. 2005;5:930–42. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- 22.Moorman PG, Hamilton RJ. Statins and cancer risk: what do we know and where do we go from here? Epidemiology. 2007;18:194–6. doi: 10.1097/01.ede.0000254699.31405.e2. [DOI] [PubMed] [Google Scholar]
- 23.Coogan PF, Rosenberg L, Palmer JR, Strom BL, Zauber AG, Shapiro S. Statin use and the risk of breast and prostate cancer. Epidemiology. 2002;13:262–7. doi: 10.1097/00001648-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Platz EA, Leitzmann MF, Visvanathan K, Rimm EB, et al. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst. 2006;98:1819–25. doi: 10.1093/jnci/djj499. [DOI] [PubMed] [Google Scholar]
- 25.Hong MY, Seeram NP, Zhang Y, Heber D. Chinese red yeast rice versus lovastatin effects on prostate cancer cells with and without androgen receptor overexpression. J Med Food. 2008;11:657–66. doi: 10.1089/jmf.2007.0702. [DOI] [PubMed] [Google Scholar]
- 26.Culig Z, Hoffmann J, Erdel M, et al. Switch from antagonist to agonist of the androgen receptor bicalutamide is associated with prostate tumour progression in a new model system. Br J Cancer. 1999;81:242–51. doi: 10.1038/sj.bjc.6690684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devgan SA, Henderson BE, Yu MC, et al. Genetic variation of 3 beta-hydroxysteroid dehydrogenase type II in three racial/ethnic groups: implications for prostate cancer risk. Prostate. 1997;33:9–12. doi: 10.1002/(sici)1097-0045(19970915)33:1<9::aid-pros2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 28.Fung KM, Samara EN, Wong C, et al. Increased expression of type 2 3alpha-hydroxysteroid dehydrogenase/type 5 17beta-hydroxysteroid dehydrogenase (AKR1C3) and its relationship with androgen receptor in prostate carcinoma. Endocr Relat Cancer. 2006;13:169–80. doi: 10.1677/erc.1.01048. [DOI] [PubMed] [Google Scholar]
- 29.Torres JM, Ruiz E, Ortega E. Development of a quantitative RT-PCR method to study 5alpha-reductase mRNA isozymes in rat prostate in different androgen status. Prostate. 2003;56:74–9. doi: 10.1002/pros.10221. [DOI] [PubMed] [Google Scholar]
- 30.Stanbrough M, Bubley GJ, Ross K, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–25. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 31.Steers WD. 5alpha-reductase activity in the prostate. Urology. 2001;58:17–24. doi: 10.1016/s0090-4295(01)01299-7. [DOI] [PubMed] [Google Scholar]
- 32.Thompson IM, Goodman PJ, Tangen CM, et al. The Influence of Finasteride on the Development of Prostate Cancer. N Engl J Med. 2003;349:215–24. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 33.Gleave M, Hsieh JT, Gao CA, von Eschenbach AC, Chung LW. Acceleration of human prostate cancer growth in vivo by factors produced by prostate and bone fibroblasts. Cancer Res. 1991;51:3753–61. [PubMed] [Google Scholar]
- 34.Hong MY, Bancroft LK, Turner ND, et al. Fish oil decreases oxidative DNA damage by enhancing apoptosis in rat colon. Nutr Cancer. 2005;52:166–75. doi: 10.1207/s15327914nc5202_7. [DOI] [PubMed] [Google Scholar]
- 35.Holt PR, Moss SF, Kapetanakis AM, Petrotos A, Wang S. Is Ki-67 a better proliferative marker in the colon than proliferating cell nuclear antigen? Cancer Epidemiol Biomarkers Prev. 1997;6:131–5. [PubMed] [Google Scholar]
- 36.Hong MY, Seeram NP, Zhang Y, Heber D. Anti-cancer effects of Chinese red yeast rice versus monacolin K alone on colon cancer cells. J Nutr Biochem. 2008;19:448–58. doi: 10.1016/j.jnutbio.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hall SA, Page ST, Travison TG, Montgomery RB, Link CL, McKinlay JB. Do statins affect androgen levels in men? Results from the Boston area community health survey. Cancer Epidemiol Biomarkers Prev. 2007;16:1587–94. doi: 10.1158/1055-9965.EPI-07-0306. [DOI] [PubMed] [Google Scholar]
- 38.Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J Clin Invest. 2005;115:959–68. doi: 10.1172/JCI200519935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solomon KR, Freeman MR. Do the cholesterol-lowering properties of statins affect cancer risk? Trends Endocrinol Metab. 2008;19:113–21. doi: 10.1016/j.tem.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 40.Mondul AM, Clipp SL, Helzlsouer KJ, Platz EA. Association between plasma total cholesterol concentration and incident prostate cancer in the CLUE II cohort. Cancer Causes Control. 2010;21:61–8. doi: 10.1007/s10552-009-9434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pelletier G, Luu-The V, El-Alfy M, Li S, Labrie F. Immunoelectron microscopic localization of 3ß-hydroxysteroid dehydrogenase and type 5 17ß-hydroxysteroid dehydrogenase in the human prostate and mammary gland. J Mol Endocrinol. 2001;26:11–9. doi: 10.1677/jme.0.0260011. [DOI] [PubMed] [Google Scholar]
- 42.Hong MY, Seeram NP, Heber D. Pomegranate polyphenols down-regulate expression of androgen-synthesizing genes in human prostate cancer cells overexpressing the androgen receptor. J Nutr Biochem. 2008;19:848–55. doi: 10.1016/j.jnutbio.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Q, Li W, Zhang Y, et al. Androgen receptor regulates a distinct transcription program inandrogen-independent prostate cancer. Cell. 2009;138:245–56. doi: 10.1016/j.cell.2009.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nature Rev. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 45.Gelmann EP. Molecular biology of the androgen receptor. J Clin Oncol. 2002;20:3001–15. doi: 10.1200/JCO.2002.10.018. [DOI] [PubMed] [Google Scholar]
- 46.Grossman ME, Huang H, Tindall DJ. Androgen receptor signaling in androgen refractory prostate cancer. J Natl Cancer Inst. 2001;93:1687–97. doi: 10.1093/jnci/93.22.1687. [DOI] [PubMed] [Google Scholar]
- 47.Graaf MR, Richel DJ, van Noorden CJ, Guchelaar HJ. Effects of statins and farnesyltransferase inhibitors on the development and progression of cancer. Cancer Treat Rev. 2004;30:609–41. doi: 10.1016/j.ctrv.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 48.Mo H, Elson CE. Studies of the isoprenoid-mediated inhibition of mevalonate synthesis applied to cancer chemotherapy and chemoprevention. Exp Biol Med. 2004;229:567–85. doi: 10.1177/153537020422900701. [DOI] [PubMed] [Google Scholar]
- 49.Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J Clin Invest. 2005;115:959–68. doi: 10.1172/JCI200519935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu J, Zhang J, Shi Y, Grimsgaard S, Alraek T, Fønnebø V. Chinese red yeast rice (Monascus purpureus) for primary hyperlipidemia: a meta-analysis of randomized controlled trials. Chin Med. 2006;1:4–16.51. doi: 10.1186/1749-8546-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Venero CV, Venero JV, Wortham DC, Thompson PD. Lipid-lowering efficacy of red yeast rice in a population intolerant to statins. Am J Cardiol. 2010;105:664–6. doi: 10.1016/j.amjcard.2009.10.045. [DOI] [PubMed] [Google Scholar]