Abstract
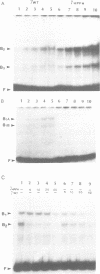
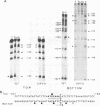
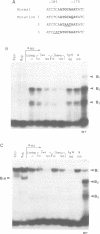
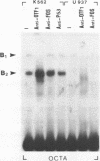
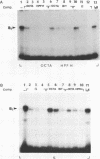
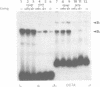
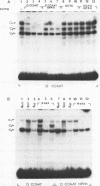
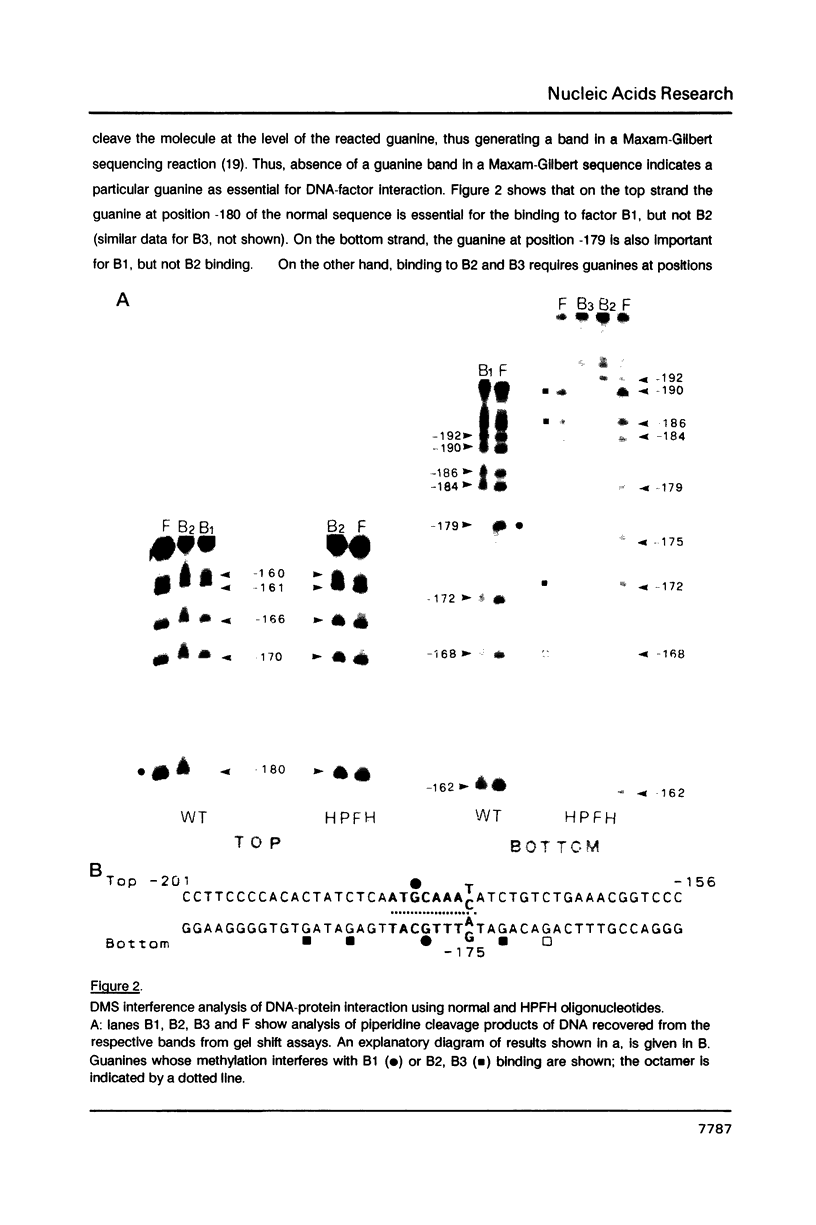
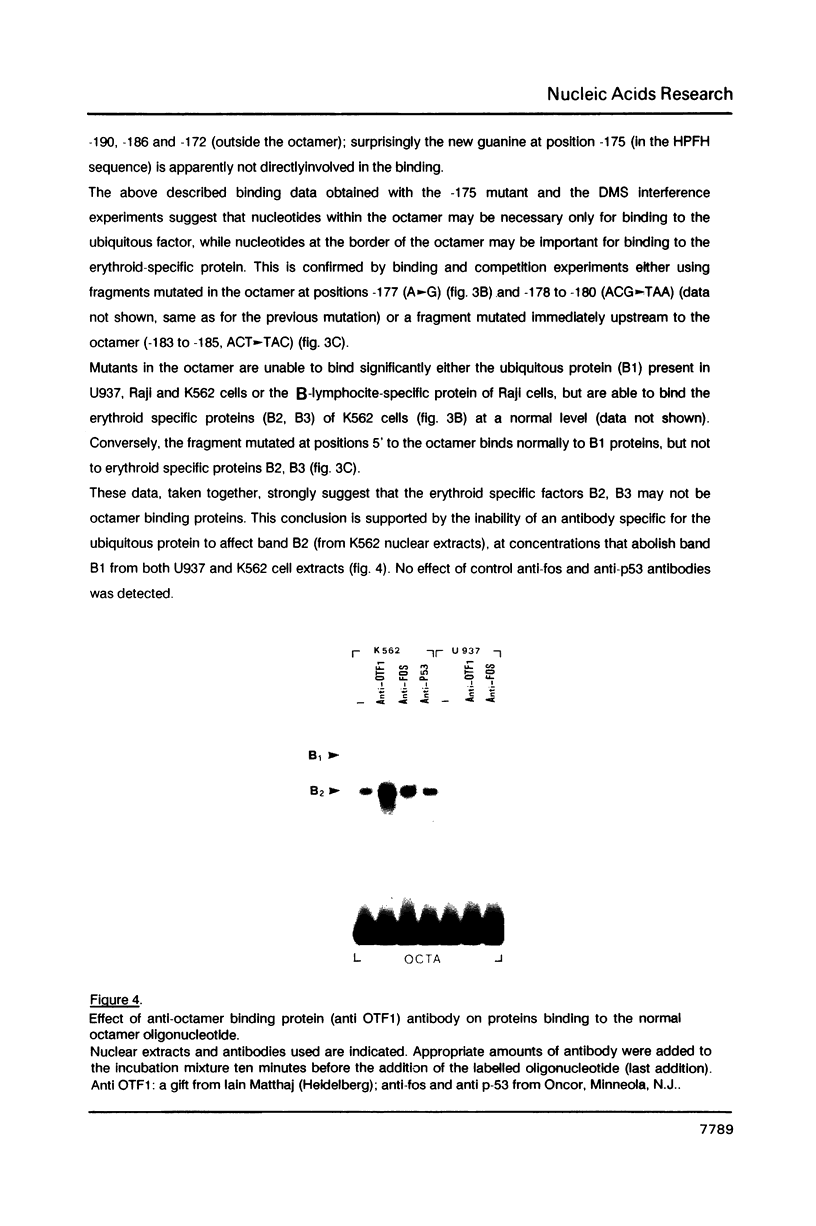
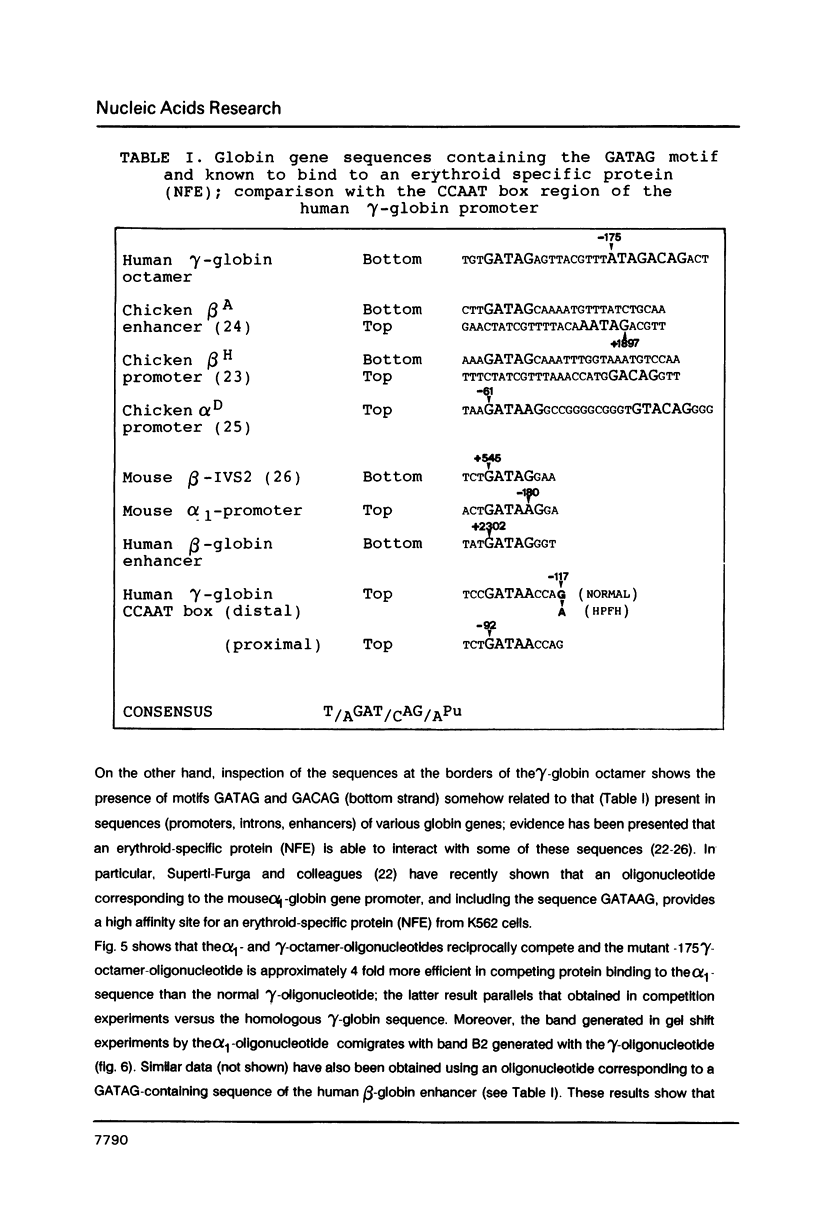
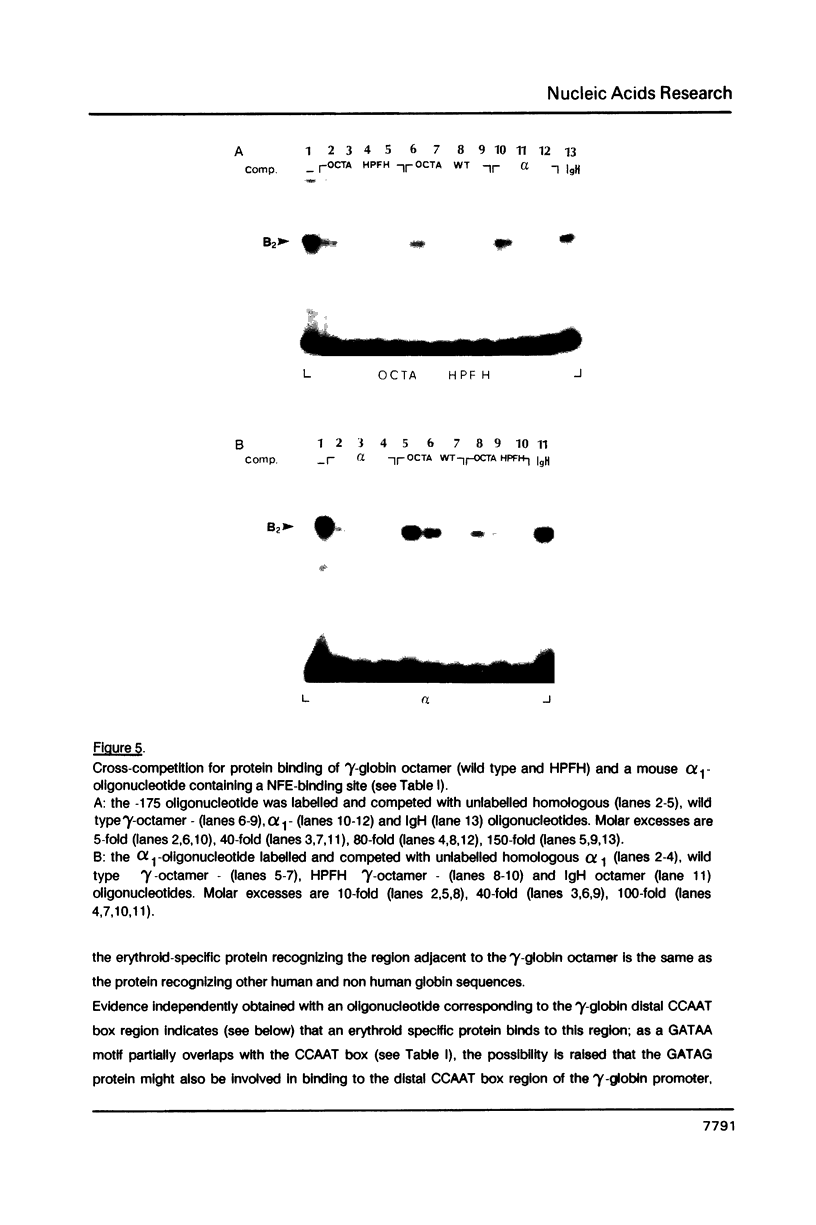
Genetic evidence indicates that single point mutations in the gamma-globin promoter may be the cause of high expression of the mutated gene in the adult period (Hereditary Persistence of Fetal Hemoglobin, HPFH). Here we show that one of these mutations characterized by a T----C substitution at position -175 in a conserved octamer (ATGCAAAT) sequence, abolishes the ability of a ubiquitous octamer binding nuclear protein to bind a gamma-globin promoter fragment containing the mutated sequence; however, the ability of two erythroid specific proteins to bind the same fragment is increased three to five fold. DMS interference and binding experiments with mutated fragments indicate that the ubiquitous protein recognizes the octamer sequence, while the erythroid specific proteins B2, B3 recognize flanking nucleotides. Competition experiments indicate that protein B2 corresponds to an erythroid-specific protein known to bind to a consensus GATAG sequence present at several locations in alpha, beta and gamma-globin genes. Although the distal CCAAT box region of the gamma-globin gene shows a related sequence, an oligonucleotide including this sequence does not show any ability to bind the above mentioned erythroid protein; instead, it binds a different erythroid specific protein, in addition to a ubiquitous protein. The -117 G----A mutation also known to cause HPFH, and mapping two nucleotides upstream from the CCAAT box, greatly decreases the binding of the erythroid-specific, but not that of the ubiquitous protein, to the CCAAT box region fragment.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collins F. S., Boehm C. D., Waber P. G., Stoeckert C. J., Jr, Weissman S. M., Forget B. G., Kazazian H. H., Jr Concordance of a point mutation 5' to the G gamma globin gene with G gamma beta +. Hereditary persistence of fetal hemoglobin in the black population. Blood. 1984 Dec;64(6):1292–1296. [PubMed] [Google Scholar]
- Collins F. S., Metherall J. E., Yamakawa M., Pan J., Weissman S. M., Forget B. G. A point mutation in the A gamma-globin gene promoter in Greek hereditary persistence of fetal haemoglobin. Nature. 1985 Jan 24;313(6000):325–326. doi: 10.1038/313325a0. [DOI] [PubMed] [Google Scholar]
- Collins F. S., Stoeckert C. J., Jr, Serjeant G. R., Forget B. G., Weissman S. M. G gamma beta+ hereditary persistence of fetal hemoglobin: cosmid cloning and identification of a specific mutation 5' to the G gamma gene. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4894–4898. doi: 10.1073/pnas.81.15.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson B. M., Nickol J. M., Jackson P. D., Felsenfeld G. Analysis of the tissue-specific enhancer at the 3' end of the chicken adult beta-globin gene. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4786–4790. doi: 10.1073/pnas.84.14.4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher C., Heintz N., Roeder R. G. Purification and characterization of OTF-1, a transcription factor regulating cell cycle expression of a human histone H2b gene. Cell. 1987 Dec 4;51(5):773–781. doi: 10.1016/0092-8674(87)90100-0. [DOI] [PubMed] [Google Scholar]
- Galson D. L., Housman D. E. Detection of two tissue-specific DNA-binding proteins with affinity for sites in the mouse beta-globin intervening sequence 2. Mol Cell Biol. 1988 Jan;8(1):381–392. doi: 10.1128/mcb.8.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas R., Bender M., Lotshaw C., Waber P., Kazazian H., Jr, Stamatoyannopoulos G. Chinese A gamma fetal hemoglobin: C to T substitution at position-196 of the A gamma gene promoter. Blood. 1986 Jun;67(6):1777–1779. [PubMed] [Google Scholar]
- Gelinas R., Endlich B., Pfeiffer C., Yagi M., Stamatoyannopoulos G. G to A substitution in the distal CCAAT box of the A gamma-globin gene in Greek hereditary persistence of fetal haemoglobin. Nature. 1985 Jan 24;313(6000):323–325. doi: 10.1038/313323a0. [DOI] [PubMed] [Google Scholar]
- Giglioni B., Casini C., Mantovani R., Merli S., Comi P., Ottolenghi S., Saglio G., Camaschella C., Mazza U. A molecular study of a family with Greek hereditary persistence of fetal hemoglobin and beta-thalassemia. EMBO J. 1984 Nov;3(11):2641–2645. doi: 10.1002/j.1460-2075.1984.tb02187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper B., Jackson P. D., Felsenfeld G. Protein-binding sites within the 5' DNase I-hypersensitive region of the chicken alpha D-globin gene. Mol Cell Biol. 1987 Jun;7(6):2059–2069. doi: 10.1128/mcb.7.6.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani R., Malgaretti N., Giglioni B., Comi P., Cappellini N., Nicolis S., Ottolenghi S. A protein factor binding to an octamer motif in the gamma-globin promoter disappears upon induction of differentiation and hemoglobin synthesis in K562 cells. Nucleic Acids Res. 1987 Nov 25;15(22):9349–9364. doi: 10.1093/nar/15.22.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani R., Malgaretti N., Nicolis S., Giglioni B., Comi P., Cappellini N., Bertero M. T., Caligaris-Cappio F., Ottolenghi S. An erythroid specific nuclear factor binding to the proximal CACCC box of the beta-globin gene promoter. Nucleic Acids Res. 1988 May 25;16(10):4299–4313. doi: 10.1093/nar/16.10.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Ottolenghi S., Giglioni B., Pulazzini A., Comi P., Camaschella C., Serra A., Guerrasio A., Saglio G. Sardinian delta beta zero-thalassemia: a further example of a C to T substitution at position -196 of the A gamma globin gene promoter. Blood. 1987 Apr;69(4):1058–1061. [PubMed] [Google Scholar]
- Ottolenghi S., Nicolis S., Taramelli R., Malgaretti N., Mantovani R., Comi P., Giglioni B., Longinotti M., Dore F., Oggiano L. Sardinian G gamma-HPFH: a T----C substitution in a conserved "octamer" sequence in the G gamma-globin promoter. Blood. 1988 Mar;71(3):815–817. [PubMed] [Google Scholar]
- Parslow T. G., Jones S. D., Bond B., Yamamoto K. R. The immunoglobulin octanucleotide: independent activity and selective interaction with enhancers. Science. 1987 Mar 20;235(4795):1498–1501. doi: 10.1126/science.3029871. [DOI] [PubMed] [Google Scholar]
- Plumb M. A., Lobanenkov V. V., Nicolas R. H., Wright C. A., Zavou S., Goodwin G. H. Characterisation of chicken erythroid nuclear proteins which bind to the nuclease hypersensitive regions upstream of the beta A- and beta H-globin genes. Nucleic Acids Res. 1986 Oct 10;14(19):7675–7693. doi: 10.1093/nar/14.19.7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruijn G. J., van Driel W., van Miltenburg R. T., van der Vliet P. C. Promoter and enhancer elements containing a conserved sequence motif are recognized by nuclear factor III, a protein stimulating adenovirus DNA replication. EMBO J. 1987 Dec 1;6(12):3771–3778. doi: 10.1002/j.1460-2075.1987.tb02712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosales R., Vigneron M., Macchi M., Davidson I., Xiao J. H., Chambon P. In vitro binding of cell-specific and ubiquitous nuclear proteins to the octamer motif of the SV40 enhancer and related motifs present in other promoters and enhancers. EMBO J. 1987 Oct;6(10):3015–3025. doi: 10.1002/j.1460-2075.1987.tb02607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidereit C., Heguy A., Roeder R. G. Identification and purification of a human lymphoid-specific octamer-binding protein (OTF-2) that activates transcription of an immunoglobulin promoter in vitro. Cell. 1987 Dec 4;51(5):783–793. doi: 10.1016/0092-8674(87)90101-2. [DOI] [PubMed] [Google Scholar]
- Singh H., Sen R., Baltimore D., Sharp P. A. A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature. 1986 Jan 9;319(6049):154–158. doi: 10.1038/319154a0. [DOI] [PubMed] [Google Scholar]
- Staudt L. M., Singh H., Sen R., Wirth T., Sharp P. A., Baltimore D. A lymphoid-specific protein binding to the octamer motif of immunoglobulin genes. Nature. 1986 Oct 16;323(6089):640–643. doi: 10.1038/323640a0. [DOI] [PubMed] [Google Scholar]
- Surrey S., Delgrosso K., Malladi P., Schwartz E. A single-base change at position -175 in the 5'-flanking region of the G gamma-globin gene from a black with G gamma-beta+ HPFH. Blood. 1988 Mar;71(3):807–810. [PubMed] [Google Scholar]
- Tate V. E., Wood W. G., Weatherall D. J. The British form of hereditary persistence of fetal hemoglobin results from a single base mutation adjacent to an S1 hypersensitive site 5' to the A gamma globin gene. Blood. 1986 Dec;68(6):1389–1393. [PubMed] [Google Scholar]
- Waber P. G., Bender M. A., Gelinas R. E., Kattamis C., Karaklis A., Sofroniadou K., Stamatoyannopoulos G., Collins F. S., Forget B. G., Kazazian H. H., Jr Concordance of a point mutation 5' to the A gamma-globin gene with A gamma beta + hereditary persistence of fetal hemoglobin in Greeks. Blood. 1986 Feb;67(2):551–554. [PubMed] [Google Scholar]