Abstract
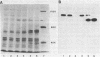
Protein synthesis elongation factor 2 (EF-2) from all archaebacteria so far analysed, is susceptible to inactivation by diphtheria toxin, a property which it shares with EF-2 from the eukaryotic 8OS translation system. To resolve the structural basis of diphtheria toxin susceptibility, the structural gene for the EF-2 from an archaebacterium, Methanococcus vannielii, was cloned and its nucleotide sequence determined. It was found that (i) this gene is closely linked to that coding for elongation factor 1 alpha-(EF-1 alpha), (ii) the size of the gene product, as derived from the nucleotide sequence, lies between those for EF-2 from eukaryotes and eubacteria, (iii) it displays a higher sequence similarity to eukaryotic EF-2 than to eubacterial homologues, and (iv) the histidine residue which is modified to diphthamide and then ADP-ribosylated by diphtheria toxin is present in a sequence context similar to that of eukaryotic EF-2 but it is not conserved in eubacterial EF-G. The EF-2 gene from Methanococcus is expressed in transformed Saccharomyces cerevisiae but is not ADP-ribosylated by diphtheria toxin. This indicates that the Saccharomyces enzyme system is unable to post-translationally convert the respective histidine residue from the Methanococcus EF-2 into diphthamide.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammerer G. Expression of genes in yeast using the ADCI promoter. Methods Enzymol. 1983;101:192–201. doi: 10.1016/0076-6879(83)01014-9. [DOI] [PubMed] [Google Scholar]
- Botstein D., Falco S. C., Stewart S. E., Brennan M., Scherer S., Stinchcomb D. T., Struhl K., Davis R. W. Sterile host yeasts (SHY): a eukaryotic system of biological containment for recombinant DNA experiments. Gene. 1979 Dec;8(1):17–24. doi: 10.1016/0378-1119(79)90004-0. [DOI] [PubMed] [Google Scholar]
- Brown B. A., Bodley J. W. Primary structure at the site in beef and wheat elongation factor 2 of ADP-ribosylation by diphtheria toxin. FEBS Lett. 1979 Jul 15;103(2):253–255. doi: 10.1016/0014-5793(79)81339-3. [DOI] [PubMed] [Google Scholar]
- Dever T. E., Glynias M. J., Merrick W. C. GTP-binding domain: three consensus sequence elements with distinct spacing. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1814–1818. doi: 10.1073/pnas.84.7.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrmann R., Henschen A., Klink F. Primary structure of elongation factor 2 around the site of ADP-ribosylation is highly conserved from archaebacteria to eukaryotes. FEBS Lett. 1985 Jun 3;185(1):37–42. doi: 10.1016/0014-5793(85)80736-5. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Kohno K., Uchida T., Ohkubo H., Nakanishi S., Nakanishi T., Fukui T., Ohtsuka E., Ikehara M., Okada Y. Amino acid sequence of mammalian elongation factor 2 deduced from the cDNA sequence: homology with GTP-binding proteins. Proc Natl Acad Sci U S A. 1986 Jul;83(14):4978–4982. doi: 10.1073/pnas.83.14.4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landsmann J., Kröger M., Hobom G. The rex region of bacteriophage lambda: two genes under three-way control. Gene. 1982 Nov;20(1):11–24. doi: 10.1016/0378-1119(82)90083-x. [DOI] [PubMed] [Google Scholar]
- Nomura M., Gourse R., Baughman G. Regulation of the synthesis of ribosomes and ribosomal components. Annu Rev Biochem. 1984;53:75–117. doi: 10.1146/annurev.bi.53.070184.000451. [DOI] [PubMed] [Google Scholar]
- Ohama T., Yamao F., Muto A., Osawa S. Organization and codon usage of the streptomycin operon in Micrococcus luteus, a bacterium with a high genomic G + C content. J Bacteriol. 1987 Oct;169(10):4770–4777. doi: 10.1128/jb.169.10.4770-4777.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr, Dunlop P. C., Adolph K. W., Bodley J. W. Occurrence of diphthamide in archaebacteria. J Bacteriol. 1983 Mar;153(3):1342–1347. doi: 10.1128/jb.153.3.1342-1347.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Van Ness B. G., Howard J. B., Bodley J. W. ADP-ribosylation of elongation factor 2 by diphtheria toxin. NMR spectra and proposed structures of ribosyl-diphthamide and its hydrolysis products. J Biol Chem. 1980 Nov 25;255(22):10710–10716. [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zengel J. M., Archer R. H., Lindahl L. The nucleotide sequence of the Escherichia coli fus gene, coding for elongation factor G. Nucleic Acids Res. 1984 Feb 24;12(4):2181–2192. doi: 10.1093/nar/12.4.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]