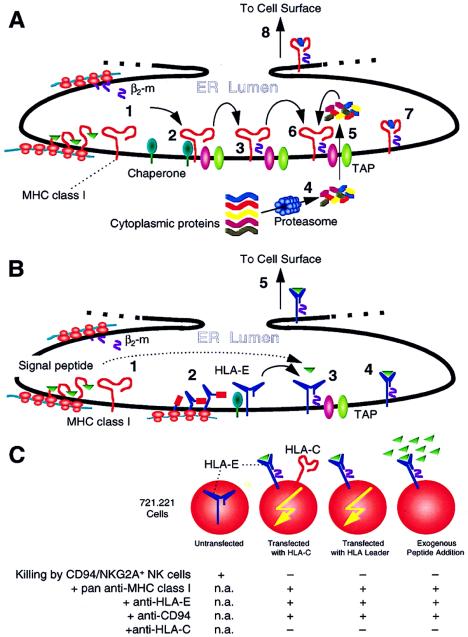
Figure 1.
(A) Synthesis and assembly of classical MHC class I molecules. Nascent MHC class I heavy (MHCI) chains and β2-microglobulin (β2-m) are generated in the endoplasmic reticulum (ER) lumen (step 1). The heavy chain initially associates with several different chaperone molecules (not shown for simplicity) (step 2). Upon assembly with β2-m, the MHCI-β2-m complex dissociates from the chaperones and associates with TAP molecules (step 3), which facilitate the transport of proteasome-degraded (step 4) small peptides across the ER membrane (step 5). TAP also has other assembly functions. The peptides then bind to the peptide binding cleft of MHCI (step 6), forming stable complexes that dissociate from TAP (step 7) and are transported to the cell surface (step 8). If assembly is disrupted, such as with TAP deficiency (as in the TAP-deficient RMA-S mouse cell), MHCI-β2-m molecules do not form intact stable complexes on the cell surface. The complexes can be stabilized by addition of exogenous peptides, but only if these peptides can bind MHCI (see ref. 27). (B) Synthesis and assembly of HLA-E (and Qa-1) molecules. In contrast to classical MHC class I molecules, HLA-E and Qa-1 bind peptides that are derived from the signal peptides of classical MHC class I molecules. The details of HLA-E synthesis and assembly are forthcoming but presumably should otherwise resemble those of classical MHC class I production, as initial studies indicate similarities (28, 29). Nascent MHCI and β2-m are generated in the ER lumen (step 1). In addition, nascent HLA-E heavy chains are also produced that associate with chaperones (step 2). Upon assembly with β2-m, MHCI-β2-m complex dissociates from chaperones and associates with TAP molecules (step 3) that are required, perhaps because of chaperone/assembly functions other than peptide transport. Fragments of signal peptides from classical MHC class I heavy chains then bind to the peptide binding cleft of HLA-E, forming stable complexes that dissociate from TAP (step 4) and are transported to the cell surface (step 5). If assembly is disrupted, such as with a deficiency in peptide supply because of lack of the classical MHC class I signal peptides, HLA-E-β2-m molecules do not form stable complexes on the cell surface. (Such is the case for 721.221 cells.) These complexes can be stabilized by addition of exogenous peptides only if these peptides can bind HLA-E, such as leader peptides from classical MHC class I molecules. Thus, HLA-E expression requires normal HLA-E and classicalHLA class I molecules and intact assembly pathways. (C) Transfection of 721.221 cells with leader sequences of HLA class I molecules renders resistance to killing by CD94/NKG2A+ NK cells. 721.221 cells express HLA-E transcripts but no stable HLA-E molecules on the surface because the cells lack classical HLA class I molecules due to a mutation. When HLA-C (and its leader) is transfected, HLA-E is also expressed, resulting in resistance to killing by CD94/NKG2A+ NK cells (6, 32, 35). The resistance is reversed (killing occurs) with antibodies that cross-react with HLA-E or CD94/NKG2A. If the leader sequence alone is transfected (as part of HLA-E, for example), resistance still occurs despite absence of HLA-C. Moreover, addition of exogenous peptides, representing the leader sequence of classical HLA class I molecules, stabilizes otherwise unstable HLA-E-β2m complexes and renders resistance. An anti-HLA-C specific antibody does not reverse resistance in any situation. Finally, soluble HLA-E molecules bind CD94/NKG2A molecules (not depicted) (32). The data therefore indicate a specific and direct interaction between HLA-E and CD94/NKG2A. n.a., Not applicable.