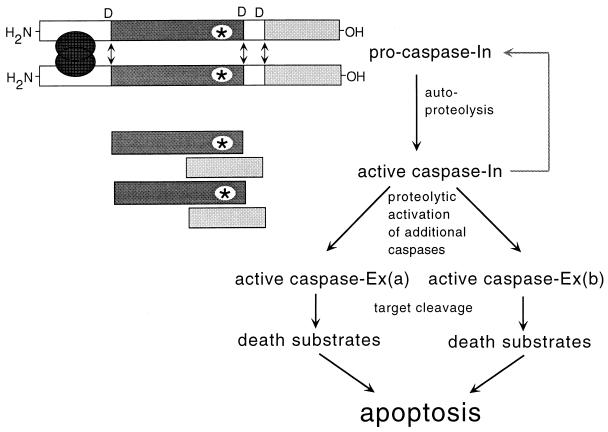
Figure 1.
Cell killing by pro-caspase aggregation. Caspases are synthesized as inactive pro-enzymes. When pro-caspases are brought into close proximity, they can self-activate through internal proteolysis that occurs after specific aspartic acid (D) residues. These cleavages result in the removal of an inhibitory N-terminal domain and the formation of a small and large subunit. Two small and two large subunits associate with each other to form the active heterotetrameric enzyme. The activated “initiator caspase” (caspase-In) can proteolytically process and activate additional pro-caspase molecules, including downstream “executional caspases” (caspase-Ex), thereby initiating a caspase cascade. The activity of this cascade leads to the proteolysis of a variety of specific target proteins and mediates the coordinate destruction of organelles and macromolecules during apoptosis. The aggregation of pro-caspases is normally regulated by adapter molecules that interact with the prodomain, but recombinant molecules have been constructed that can be aggregated by small, lipid-permeable chemicals.