Fig. 3. Activation of EGFR in cell lines and patients with acquired resistance to crizotinib.
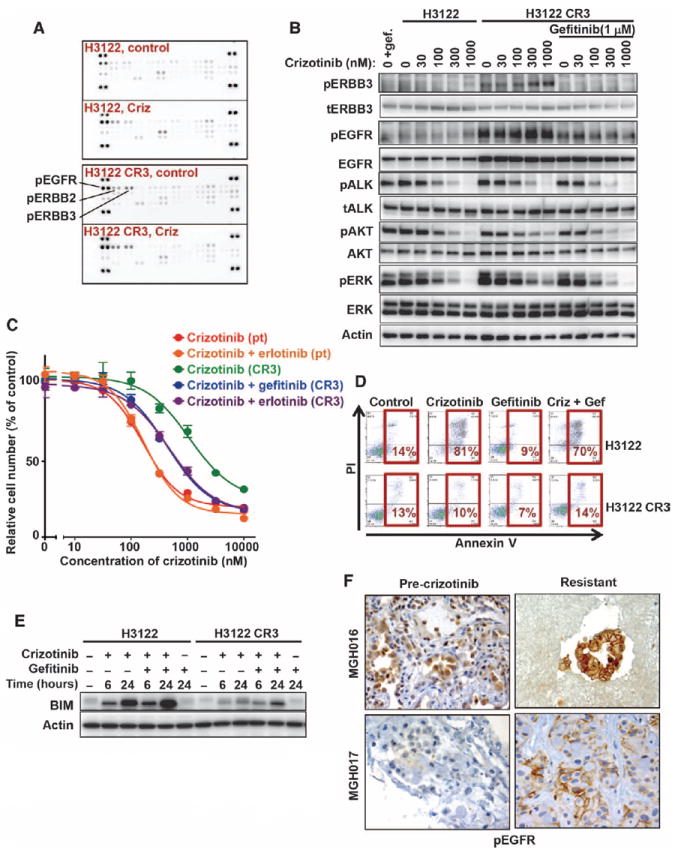
(A) Parental H3122 and H3122 CR3 cells were incubated in the absence (control) or presence (Criz) of 1 μM crizotinib for 6 hours, and lysates were incubated with phospho-RTK arrays (R&D Systems). The positions of phospho-EGFR, phospho-ERBB2, and phospho-ERBB3 are indicated. (B) H3122 CR3 cells were treated for 6 hours with the indicated concentrations of crizotinib in the presence or absence of gefitinib. Cell lysates were probed with the indicated antibodies. (C) Sensitization of resistant H3122 CR3 cells by treatment with both crizotinib and an EGFR TKI (gefitinib or erlotinib). Parental H3122 and H3122 CR3 cells were treated with the indicated doses of crizotinib in the presence or absence of 2 μM gefitinib or 1 μM erlotinib for 72 hours. Cell survival was determined using the CellTiter-Glo viability assay. Each concentration was measured in sextuplicate, and the average and SD are shown. (D) Lack of apoptosis induction in H3122 CR3 cells treated with crizotinib (Criz) and gefitinib (Gef). Parental H3122 and H3122 CR3 cells were treated with 1 μM crizotinib, 2 μM gefitinib, or the combination. After 72 hours, cells were stained with Alexa Fluor 633–labeled annexin V and PI and analyzed by flow cytometry. The percentage of cells undergoing apoptosis is shown. (E) Defective up-regulation of BIM in H3122 CR3 cells treated with crizotinib and gefitinib. H3122 and H3122 CR3 cells were treated with 1 μM crizotinib, 2 μM gefitinib, or both drugs for 6 or 24 hours. Lysates were probed with BIM and actin-specific antibodies. (F) Increased EGFR activation in crizotinib-resistant tumors. Pre-crizotinib and crizotinib-resistant tumors were stained using a phospho-EGFR–specific antibody. Shown are two cases, MGH016 and MGH017, both of which demonstrate stronger plasma membrane staining of phospho-EGFR in the resistant cancer than in the corresponding pre-crizotinib sample.
