Abstract
Background:
The cutaneous leishmaniasis (CL) has been occurred in Dehbakri County, located 46 km of Bam District, Kerman Province since 2004–2005. Phlebotomus papatasi is an important vector of zoonotic cutaneous leishmanisis (ZCL) as well as sand fly fever and P. sergenti is considered as main vector of anthroponotic cutaneous leishmaniasis (ACL) in Iran. There are several measures for vector control with emphasizing on insecticides. The objective of this study was to determine the baseline susceptibility of leishmaniasis vectors to the DDT and deltamethrin in an endemic focus of CL in southern Iran.
Methods:
Baseline susceptibility tests were carried out on field collected strains of P. papatasi and P. sergenti and tested with WHO impregnated papers with DDT 4.0% and deltamethrin 0.05% in the focus of disease in Dehbakri County during summer 2010. The values of LT50 and LT90 were determined using probit analysis and regression lines.
Results:
The LT50 value of DDT 4.0% and deltamethrin 0.05% against P. papatasi was 20.6 and 13.6 minutes respectively. The same data for P. sergenti were ranged between 21.8 and 17.7 minutes.
Conclusion:
The results of tests will provide a guideline for implementation of vector control using pesticides such as impregnated bed nets, indoor residual spraying and fogging.
Keywords: Phlebotomus papatasi, Phlebotomus sergenti, insecticides, susceptibility, Iran
Introduction
Phlebotomine sand flies are known to transmit varieties of zoonotic diseases including leishmaniases (protozoa), bartonellosis (bacteria) and sand fly fever (viruses) that affect humans and animals in 88 countries worldwide (Verani et al. 1991, Saidi et al. 1997, Tesh et al. 1997, Mehrabi Tavana 2001, Sharma and Singh 2008, Depaquit et al. 2010). Phlebotomus papatasi is widely distributed throughout the world. It is a common species in the Eastern Mediterranean region and considered as important vector of zoonotic cutaneous leishmaniasis as well as sand fly fever in Iran. Cutaneous Leishmaniasis (CL) is reported from 50% of the 31 provinces in Iran (Yaghoobi-Ershadi et al. 1999, Rassi et al. 2004, Rassi et al. 2006, Rassi et al. 2007, Rassi et al. 2011). The activity of P. papatasi and P. sergenti in Bam starts from late May and extends to mid October with two peaks, one in mid or late June and the second in early or mid September. Phlebotomus papatasi could be a Leishmania vector for humans and gerbils and is susceptible to DDT (Yaghoobi-Ershadi and Akhavan 1999). Leishmaniasis is endemic in at least 88 poor countries, with the global prevalence of all forms of the disease is 12 million, with 1.5–2 million cases of CL and half a million cases of visceral form.
Current control measures, including environmental sanitation and drug treatment of cases, are expensive and cannot be sustained effectively by poor countries due to the problems of financing and implementation. Moreover, toxicity associated with some of the most widely available drug treatments, including injections of pentavalent antimony compounds, and the resistance developed by the parasite underline the need for development of effective methods of prevention, especially vaccines (Noazin et al. 2008, Noazin et al. 2009). The findings indicate that the whole-parasite vaccine candidates tested do not confer significant protection against human leishmaniasis.
Cutaneous leishmaniasis is still an important public health problem in many parts of the world, where 90% of the cases occur in seven countries including Iran. Two main species of CL are present in Iran; anthroponotic CL (ACL) due to L. tropica is the predominant species in many large and medium size cities. The city of Bam has been endemic for many years and a well-known focus for ACL, where recent field trials of autoclaved L. major vaccine against ACL were conducted. Zoonotic CL (ZCL) caused by L. major, is found in many rural foci in the north, east and south of the country (Sharifi et al. 2010). Emergence or re-emergence of CL has recently occurred in many countries. Both ZCL and ACL have been prevalent in a number of rural and urban areas of Iran. They have also emerged in new foci during recent decades (Fazaeli et al. 2009).
A new emerging focus of ACL due to L. tropica in rural areas of Dehbakri County, south-eastern Iran, after the earthquake of 2003 has been confirmed using polymerase chain reaction (PCR). The current emergence was unexpected in this rural area, where no previous history of ACL was recorded. According to our knowledge this is the first report of a gradually establishing new ACL focus in rural communities after the 2003 earthquake (Sharifi et al. 2011). Nested PCR revealed the first report of occurrence of ACL due to L. tropica among 3516 inhabitants in Jiroft City (Pouresmailian et al. 2010).
Molecular and direct detection of Leishmania parasites revealed the occurrence of ACL in rural parts of the Bam City after earthquake. Natural disaster completely provides favorable condition for sand fly breeding places as well as propagation of L. tropica in the displaced population due to earthquake (Sharifi et al. 2011).
The fauna and monthly activity of the ACL vector has been studied in Bam using the sticky paper traps. The sand fly species identified were P. sergenti (77.25%), P. papatasi (19.00%), Sergentomyia baghdadis (1.69%), S. sintoni (1.64 %) and S. tiberiadis (0.42%). Phlebotomus sergenti was the predominant species, and formed 85.11% and 81.83% of species from the human and animal indoor habitats, respectively. Phlebotomus sergenti have two peaks of activity during the year, one of which is from the beginning of July, and the other is at the beginning of September (Aghasi and Sharifi 2003).
According to the Health Center report the main activities for sand fly control was using DDT as Indoor Residual Spraying when there was malaria problem in the region. Subsequently permethrin is used for space spraying. Deltamethrin impregnated bed nets at the dosage of 25 mg/m2 also had been employed for vector control. The complementary measure was using repellents and covering the lesion with sterile bandage.
Dehbakri County is encountered as the newly emerging ACL foci in Kerman Province since 2003 just after tremendous Bam earthquake. Following this event, some affected people were migrated to Dehbakri Country due to temperate climate compared to Bam City. The prevalence of CL was 213.8 per thousand of people during 2003–2010. In addition, the CL incidence was estimated 34.3 per thousand people at 2010 (unpublished data).
The aim of this study was the determination of baseline data on susceptibility level of P. papatasi and P. sergenti to DDT and deltamethrin in CL affected villages where the chemical intervention was remained as the main choice for CL control method in the Dehbakri focus, southest Iran.
Materials and Methods
Study area
Dehbakri County is located at the foothill of Jebal-Barez Mountain and 48 km far from southwestern of Bam District (Fig. 1). The population of Dehbakri County was 2470 in 2009. The topography of these localities is foothill with the mean altitude of 2000 m above sea level. This area has a semi-desert climate, temperate at summer and cold at winter. The mean of monthly maximum and minimum temperatures were 40 °C and −5 °C in July and Dec respectively. The total annual rainfall was 220 mm. The minimum and maximum of monthly relative humidity were 45% and 92% respectively in July and Jan.
Fig. 1.
Dehbakri Country, located in Bam District, Kerman Province, south of Iran
Sand fly collection
Sand flies were collected using aspirator from 8.00 pm till 2.00 am during summer 2010. The caught sand flies were transferred to the entomological cage and then were kept by wet towel and transported to laboratory research center for implementation of susceptibility tests.
Susceptibility test
All the susceptibility tests were carried out according to the guideline of WHO (1981). The sand flies were transferred to the exposure tubes and then the mortality was scored at interval logarithmic times and the result was read after 24 hour recovery period. During the holding time, the insects were supplied with cotton pad of water in 10% sugar solution. All tested sand flies were mounted separately using Puri's media in order to identify the species. Male and female sand flies were counted separately.
Insecticide impregnated papers
Impregnated papers with DDT 4.0% and deltamethrin 0.05%, as well as control papers were supplied by World Health Organization via Iranian Centre for Diseases Management, Ministry of Health and Medical Education of Iran.
Statistical analysis
The mortality rates were corrected according to the result of control tests using Abbott’s formula whereas the control mortality rates were ranged between 5.0–20.0% (Abbott 1925). Phlebotomus papatasi and P. sergenti were exposed to WHO standard papers of DDT 4.0% and deltamethrin 0.05% at different logarithmic exposure times and the mortalities were recorded after 24 hour and subsequently analyzed by probit analysis (Finney 1971). Goodness of fit of regression lines of P. papatasi and P. sergenti was measured through the χ2 test. LT50 and LT90 values were estimated and the slope values of the regression lines were calculated. Regression lines were plotted for LT50 values for observed probit of mortalities against exposure times using Microsoft® Excel software ver. 2007.
Results
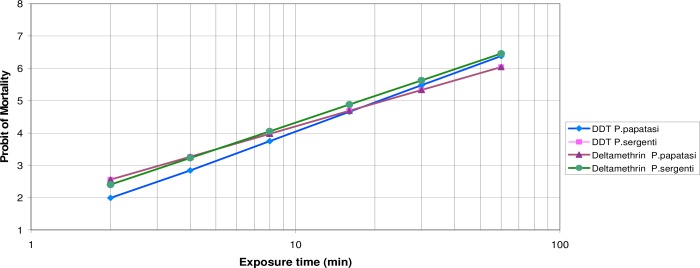
The mortality rate of field-collected P. papatasi with DDT at diagnostic concentration was 100%. The mortality rate for field-collected P. sergenti with DDT 4.0% and one hour exposure time was calculated 97.0±1%. Considering the criteria of susceptibility for mosquitoes as described by WHO, it should be noted that the tested populations of both P. papatasi and P. sergenti were also susceptible to these insecticides. The LT50 values of DDT 4.0% calculated both for P. papatasi and P. sergenti were 20.7 and 20.8 minutes. The LT90 values of DDT 4.0% were 55.6 and 75.2 minutes respectively for both latter species. The LT50 values of deltamethrin 0.05% calculated both for P. papatasi and P. sergenti were 13.6 and 17.7 minutes respectively. The LT90 values of deltamethrin 0.05% respectively were 41.9 and 52.0 minutes for P. papatasi and P. sergenti (Table 1). The regression lines of exposure times of P. papatasi and P. sergenti which exposed to WHO’s impregnated papers with DDT 4.0% and deltamethrin 0.05% are shown in Fig. 2.
Table 1.
Parameters of regression line of susceptibility of P. papatasi and P. sergenti of sand flies exposed to two insecticides
| Insecticide | Species | No of sand fly tested | Mean of Mortality (%) (diagnostic dose-1 hour) | a (y-intercept) | B (Slope) ±SE | LT50±95% C.L. | LT90±95% C.L. | X2 (Heterogeneity) calculated | X2 (Heterogeneity) table (df) | P. value |
|---|---|---|---|---|---|---|---|---|---|---|
| DDT4% | P. papatasi | 153 | 100% | −3.9736 | 3.011± 0.493 | 16.5127 | 40.1724 | 52.724 (4) | 18.47 (4) | <0.001 |
| 20.8771 | 55.6294 | |||||||||
| 26.8631 | 97.1896 | |||||||||
| DDT4% | P. sergenti | 217 | 97.0±1% | −3.1495 | 2.3547± 0.683 | 8.5907 | 32.1209 | 18.801 (4) | 13.28 (4) | <0.001 |
| 21.7519 | 76.1663 | |||||||||
| 115.6334 | NS | |||||||||
| Deltamethrin 0.05% | P. papatasi | 130 | 100% | −2.9641 | 2.6174± 0.414 | 10.1957 | 29.8310 | 5.553 (4) | 9.49 (4) | >0.05 |
| 13.5665 | 41.8909 | |||||||||
| 17.7174 | 73.3799 | |||||||||
| Deltamethrin 0.05% | P. sergenti | 212 | 100% | −3.4225 | 2.7418± 0.352 | 14.3172 | 38.4355 | 7.745(4) | 9.49 (4) | >0.05 |
| 17.7120 | 51.9639 | |||||||||
| 22.1612 | 82.2050 |
Fig. 2.
Probit regression lines of DDT 4% and deltamethrin 0.05% against P. papatasi and P. sergenti using WHO method at Bam District, southern Iran
Discussion
Prior of this study, no reports were available on the susceptibility levels of adult P. papatasi and P. sergenti at the new CL focus of Dehbakri County, Bam District, southern Iran. The susceptibility status of P. papatasi to DDT, dieldrin, malathion, fenitrothion and propoxur has been estimated in Pali and Barmer Districts of Rajasthan. The results revealed that this species was resistant to DDT but susceptible to other insecticides (Singh and Bansal 1996). In a similar study in district Bikaner, Rajasthan the results showed that this species was resistant to DDT, dieldrin and propoxur while susceptible to malathion, fenitrothion and permethrin (Bansal and Singh 1996). In Iran, the residual spraying was carried out during 1950–1968 and the houses had been treated with DDT for malaria control. The susceptibility level of P. papatasi to DDT was studied at various parts of Iran during 1985–88. Tests were carried out in the localities where the application of DDT had been discontinued since 1969. This investigation showed that P. papatasi from Isfahan was more tolerant to DDT than other parts and probably a manifestation of DDT resistance (Rashti et al. 1992). Another study was carried out at 6 villages of Isfahan Province on P. papatasi and the values of LT50 and LT90 ranged between 18.10–20.15 minutes and 43.00–63.30 minutes respectively. In the present study, the LT50 values of DDT 4.0% against P. papatasi ranged from 20.9 to55.6 minutes. The same data for DDT 4.0 against P. sergenti were ranged between 21.8–76.2 minutes which may related with endophilic habits of fore-mentioned species and exposing the sand flies to chemical used inside the houses. The latter study showed the tolerance in P. papatasi population to DDT 4.0% at 1 hour exposure time with mortality rate of 88.8% (Yaghoobi-Ershadi et al. 1995). The susceptibility status of P. papatasi had been shown at ZCL foci of Fars Province, southern Iran and the results indicating the highly susceptibility level of this species to DDT in this area (Abai et al. 1993, Rassi et al. 2000). There are several reports revealing DDT resistance at P. papatasi, P. argentipes and Sergentomyia shorti from India (Joshi et al. 1979, Mukhopadhyay, 1992, Maroli and Khoury 2004) as well as P. papatasi in Turkey (WHO 1986). In contrast, there are also some reports emphasizing to highly susceptibility levels of P. papatasi and P. argentipes at different countries (Rahman et al. 1982, Dergacheva et al. 1986, Pener et al. 1987, Kaushal 1995, Fahnmy et al. 1996).
Resistance of P. papatasi to DDT is first reported in Bihar (Joshi et al. 1979) and then in Turkey (WHO 1986). Mean of LC90 values for DDT for Egyptian and Sudanese strains of P. papatasi ranged from 0.80–1.93% (Schmidt et al. 1969). The LC50 for DDT for Sudan P. papatasi was reported as 0.9% (Qutubuddin 1964). Results obtained from susceptibility tests on field population of P. papatasi to DDT revealed that the tested population was highly susceptible to DDT as evidenced by the discriminating concentrations of times. In Iranian foci of zoonotic leishmaniasis, control of malaria with DDT yielded no effect on the incidence of leishmaniasis or the sand fly population (Seyedi-Rashti and Nadim 1974). Measures used to control adult sand fly includes application of insecticides as residual spraying at dwellings and animal shelters, space-spraying, insecticide-treated nets and curtains, and personal protection through application of repellents/insecticides to skin or fabrics. Because the breeding places of sand flies which are generally unknown, the control measures that act specifically against immature are not feasible, although the effectiveness of a few biological and chemical agents has been demonstrated in laboratory evaluations (Alexander and Maroli 2003). In some circumstances the reservoir control is also recommended. Additionally the patient treatment with anti-parasite drug has been prescribed at the acute stage of disease (Yaghoobi-Ershadi et al. 2000). Tolerance of P. papatasi to DDT has been reported from some parts of country (Rashti et al. 1992, Abai et al. 1993, Yaghoobi-Ershadi et al. 1995).
This study showed the higher susceptibility level of P. papatasi and P. sergenti to deltamethrin 0.05% (LT50=13.6–17.7 minutes) and so this insecticides could be effectively used as residual spraying or as ITNs or LLINs in order to control the CL foci of Iran. Due to epidemic condition of CL, the affected villages of Dehbakri County were sprayed with deltamethrin WP 5.0% at 25 mg ai/m2 during June 2010.
Acknowledgments
This study was financially supported from the School of Public Health, Tehran University of Medical Sciences, and Grant No. 10487. The authors would like to thank the Dermatology and Leishmaniasis Research Center, Kerman University of Medical Sciences for their close collaboration in this study. We also wish to express our sincere thanks to the Iranian Centre of Diseases Management, Ministry of Health and Medical Education for providing facilities needed for this study. The authors declare that there is no conflict of interests.
References
- Abai MR, Javadian E. Preliminary observation on the susceptibility status of Phlebotomus papatasi (Phlebotominae, Diptera) to DDT and propoxur in Neiriz District, southern I.R.Iran. Proceedings of the 11th Congress of Plant protection; 28 August–2 September 2002; Ghilan University, Rasht. 1993. p. 297. [Google Scholar]
- Abbott WS. A method of computing the effectiveness of an insecticide. J Econ Entomol. 1925;18:265–267. [PubMed] [Google Scholar]
- Aghasi M, Sharifi I. Survey of the fauna and monthly activity of the sandfly as the vectors of the cutaneous leishmaniasis in the City of Bam. J Kerman Univ Med Sci. 2003;10:85–91. [Google Scholar]
- Alexander B, Maroli M. Control of phlebotomine sandflies. Med Vet Entomol. 2003;17:1–18. doi: 10.1046/j.1365-2915.2003.00420.x. [DOI] [PubMed] [Google Scholar]
- Azizi K, Rassi Y, Javadian E, Motazedian MH, Asgari Q, Yaghoobi-Ershadi MR. First detection of Leishmania infantum in Phlebotomus (Larroussius) major (Diptera: Psychodidae) from Iran. J Med Entomol. 2008;45:726–731. doi: 10.1603/0022-2585(2008)45[726:fdolii]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Azizi K, Rassi Y, Javadian E, Motazedian MH, Rafizadeh S, Yaghoobi Ershadi MR, Mohebali M. Phlebotomus (Paraphlebotomus) alexandri: a probable vector of Leishmania infantum in Iran. Ann Trop Med Parasitol. 2006;100:63–68. doi: 10.1179/136485906X78454. [DOI] [PubMed] [Google Scholar]
- Bansal SK, Singh KV. Susceptibility status of Phlebotomus papatasi and Sergentomyia punjabaensis (Diptera: Psychodidae) to some insecticides in district Bikaner (Rajasthan) J Commun Dis. 1996;28:28–32. [PubMed] [Google Scholar]
- Cross ER, Hyams KC. The potential effect of global warming on the geographic and seasonal distribution of Phlebotomus papatasi in Southwest Asia. Environ Health Perspect. 1996;104:724–727. doi: 10.1289/ehp.96104724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross ER, Newcomb WW, Tucker CJ. Use of weather data and remote sensing to predict the geographic and seasonal distribution of Phlebotomus papatasi in Southwest Asia. Am J Trop Med Hyg. 1996;54:530–536. doi: 10.4269/ajtmh.1996.54.530. [DOI] [PubMed] [Google Scholar]
- Depaquit J, Grandadam M, Fouque F, Andry PE, Peyrefitte C. Arthropod-borne viruses transmitted by Phlebotomine sandflies in Europe: a review. Euro Surveill. 2010;15(10):195–207. [PubMed] [Google Scholar]
- Dergacheva TI, Strekova MV. Sensitiity of Phlebotomus papatasi (Scopoli) (Diptera:Pyschodidae) to DDT in the Mubarek region of the Uzbek SSR. Med Prazit Parazitarnye Bolezni. 1986;4:84–85. [PubMed] [Google Scholar]
- Fahnmy RA, Khater EI, Sawaf BE, Shehata M. Insecticide susceptibility status of field populations of sandfly Phlebotomus papatasi in the Sinai Peninsula, Egypt. World Health Organization; 1996. Unpublished document. WHO/Leish/96.38. [Google Scholar]
- Fazaeli A, Fouladi B, Sharifi I. Emergence of cutaneous leishmaniasis in a border area at south-east of Iran: an epidemiological survey. J Vector-Borne Dis. 2009;46:36–42. [PubMed] [Google Scholar]
- Finney DJ. Probit Analysis. 3rd edition. Cambridge University Press; Cambridge, UK: 1971. [Google Scholar]
- Joshi GC, Kaul SM, Wattal BL. Susceptibility of sand flies to organochlorine insecticides in Bihar (India). Further reports. J Commun Dis. 1979;11:209–213. [Google Scholar]
- Kaul SM, Wattal BL, Bhatnagar VN, Mathur KK. Preliminary observations on the susceptibility status of Phlebotomus argentipes and Phlebotomus papatasi to DDT in two districts of North Bihar, India. J Com Dis. 1978;10:208–211. [Google Scholar]
- Kaushal K, Singh K, Das RK, Rahman SJ, Sharma SK. 1995. Laboratory and field observations on the effectiveness of DDT for the control of the vector sand fly, Phlebotomus argentipes in the kala-azar endemic state of Bihar. WHO/Leish/95.36.
- Maroli M, Khoury C. Prevention and control of leishmaniasis vectors: current approaches. Parassitologia. 2004;46:211–215. [PubMed] [Google Scholar]
- Mehrabi Tavana A. The seroepidemiological studies of sand fly fever in Iran during imposed war. Iran J Publ Health. 2001;30:145–146. [Google Scholar]
- Mohebali M, Javadian E, Yaghoobi-Ershadi MR, Akhavan AA, Hajjaran H, Abaei MR. Characterization of Leishmania infection in rodents from endemic areas of the Islamic Republic of Iran. East Mediterr Health J. 2004;10:591–599. [PubMed] [Google Scholar]
- Moin-Vaziri V, Depaquit J, Yaghoobi-Ershadi MR, Oshaghi MA, Derakhshandeh-Peykar P, Ferte H, Kaltenbach M, Bargues MD, Nadim A, Javadian E, Rassi Y, Jafari R. Geographical variation in populations of Phlebotomus (Paraphlebotomus) caucasicus (Diptera: Psychodidae) in Iran. Bull Soc Pathol Exot. 2007;100:291–295. [PubMed] [Google Scholar]
- Moin-Vaziri V, Depaquit J, Yaghoobi-Ershadi MR, Oshaghi MA, Derakhshandeh-Peykar P, Ferté H, Kaltenbach M, Bargues MD, Léger N, Nadim A. Intraspecific variation within Phlebotomus sergenti Parrot (1917) (Diptera: Psychodidae) based on mtDNA sequences in Islamic Republic of Iran. Acta Trop. 2007;102:29–37. doi: 10.1016/j.actatropica.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay AK, Axena NBL, Narasimham MVV. Susceptibility status of Phlebotomus argentipes to DDT in some kala azar endemic districts of Bihar, India. 1992. pp. 1–6. WHO/CTD/VBC/92.995, WHO/Leish/92.31. [PubMed]
- Noazin S, Farrokh Modabber F, Khamesipour A, Smith PG, Moulton LH, Nasseri K, Sharifi I, Khalil H, Velez Bernali ID, Antunesj CMF, Kieny MP, Tanner M. First generation leishmaniasis vaccines: A review of field efficacy trials. Vaccine. 2008;26:6759–6767. doi: 10.1016/j.vaccine.2008.09.085. [DOI] [PubMed] [Google Scholar]
- Noazin S, Khamesipour A, Moulton LH, Tannerd M, Nasserie K, Modabberb F, Sharifig I, Khalil H, Velez Bernali ID, Antunesj CMF, Smith PG. Efficacy of killed whole-parasite vaccines in the prevention of leishmaniasis, A meta-analysis. Vaccine. 2009;27:4747–4753. doi: 10.1016/j.vaccine.2009.05.084. [DOI] [PubMed] [Google Scholar]
- Pener H, Wilamovsky A. Basline suceptibility of Phlebotomus papatasi to insecticides. Med Vet Entomol. 1987;1:147–149. doi: 10.1111/j.1365-2915.1987.tb00335.x. [DOI] [PubMed] [Google Scholar]
- Pouresmailian S, Sharifi I, Mirzai M, Zarean M, Hakimi Parizi M. Occurrence of new focus of anthroponotic cutaneous leishmaniasis (ACL) in northern part of Jirof City, Kerman Province, Iran. 7th national and 2nd Regional Congress of Parasitology and Parasitic Diseases in Iran; 6–9 November 2010; Tehran, Iran. 2010. [Google Scholar]
- Qutubuddin Q. Preliminary note on the susceptibility of Phlebotomus to insecticides. Sudan Medical Journal. 1964;3:11–15. [Google Scholar]
- Rahman SJ, Wattal BL, Mathur KK, Joshi GC, Kumar K. Susceptibility of Phlebotomus papatasi (Scopoli) to organochlorine insecticides. J Com Dis. 1982;14:122–124. [PubMed] [Google Scholar]
- Rashti MA, Panah HY, Mohamadi HS, Jedari M. Susceptibility of Phlebotomus papatasi (Diptera: Psychodidae) to DDT in some foci of cutaneous leishmaniasis in Iran. J Am Mosq Control Assoc. 1992;8:99–100. [PubMed] [Google Scholar]
- Rassi Y, Jalali M, Vatandoost H. Susceptibility status of Phlebotomus papatasi to DDT in Arsanjan County (The new focus of cutaneous leishmaniasis) in Fars province, Iran. Iran J Publ Hlth. 2000;29:21–26. [Google Scholar]
- Rassi Y, Javadian E, Jalali M, Motazedian MH, Vatandoost H. Investigation on Zoonotic Cutaneous Leishmaniasis, southern Iran. Iran J Publ Health. 2004;33:31–35. [Google Scholar]
- Rassi Y, Gassemi MM, Javadian E, Rafizadeh S, Motazedian H, Vatandoost H. Vectors and reservoirs of cutaneous leishmaniasis in Marvdasht district, southern Islamic Republic of Iran. East Mediterr Health J. 2007;3:686–693. [PubMed] [Google Scholar]
- Rassi Y, Javadian E, Amini M, Rafizadeh S, Vatandoost H, Motazedian MH. Meriones libycus is the Main reservoir of zoonotic cutaneous leishmaniasis in south Islamic Republic Iran. East Mediterr Health J. 2006;12:474–477. [PubMed] [Google Scholar]
- Rassi Y, Oshaghi MA, Mohammadi Azani S, Abaie MR, Rafizadeh S, Mohebai M, Mohtarami F, Zeinali MK. Molecular detection of Leishmania infection due to Leishmania major and Leishmania turanica in the vectors and reservoir host in Iran. Vector-borne and Zoonotic Diseases. 2011;11:145–150. doi: 10.1089/vbz.2009.0167. [DOI] [PubMed] [Google Scholar]
- Saidi S, Tesh R, Javadian E, Sahabi Z, Nadim A. Studies on the epidemiology of sand fly fever in Iran. II. The prevalence of human and animal infection with five phlebotomus fever virus serotypes in Isfahan province. Am J Trop Med Hyg. 1997;26:288–293. doi: 10.4269/ajtmh.1977.26.288. [DOI] [PubMed] [Google Scholar]
- Schmidt ML, Schmidt JR. Insecticide susceptibilities of Phlebotomus papatasi (Scopoli) From Yet and the Sudan. J Med Entomol. 1969;6:87–90. doi: 10.1093/jmedent/6.1.87. [DOI] [PubMed] [Google Scholar]
- Seyedi-Rashti MA, Nadim A. Attempts to control zoonotic cutaneous leishmaniasis in the Isfahan area, Iran. Iran J Publ Health. 1974;2:199–203. [Google Scholar]
- Sharifi I, Fekri AR, Aflatoonian MR, Khamesipour A, Mahboudi F, Dowlati Y, Nadim A, Modabber F. Leishmaniasis recidivans among school children in Bam, South-east Iran, 1994–2006. Int J Dermatol. 2010;49:557–561. doi: 10.1111/j.1365-4632.2010.04419.x. [DOI] [PubMed] [Google Scholar]
- Sharifi I, Pouresmailian S, Aflatonian MR, Hakimi Parizi M, Fekri AR, Khamesipour A. Occurrence of new focus of anthroponotic cutaneous leishmaniasis (ACL) in Bam and its suburbs after earthquake. 7th National and 2nd Regional Congress of Parasitology and Parasitic Diseases in Iran; 6–9 November 2010; Tehran, Iran. 2010. [Google Scholar]
- Sharifi I, Pursmaelian S, Aflatoonian MR, Fotouhi Ardakani R, Hakimi Parizi M, Mirzaei M, Fekri AR, Fasihi Harandi M, Khamesipour A. Emergence of a new focus of anthroponotic cutaneous leishmaniasis due to Leishmania tropica in rural communities of Bam district after the earthquake, Iran. Trop Med Int Health. 2011 doi: 10.1111/j.1365-3156.2011.02729.x. (in Press) [DOI] [PubMed] [Google Scholar]
- Sharma U, Singh S. Insect vectors of Leishmania: distribution, physiology and their control. J Vector Borne Dis. 2008;45:255–272. [PubMed] [Google Scholar]
- Singh KV, Bansal SK. Insecticide susceptibility of Phlebotomus papatasi to organochlorine, organophosphate and carbamate compounds in some arid areas of western Rajasthan. Indian J Med Res. 1996;103:91–93. [PubMed] [Google Scholar]
- Tesh R, Saidi S, Javadian E, Nadim A. Studies on the Epidemiology of Sand fly Fever in Iran. I. Virus Isolates Obtained from Phlebotomus. Am J Trop Med Hyg. 1977;26:282–287. doi: 10.4269/ajtmh.1977.26.282. [DOI] [PubMed] [Google Scholar]
- Unpublished data 2010. Leishmaniasis Research Center, Kerman University of Medical Sciences.
- Verani P, Nicoletti L, Ciufolini MG, Balducci M. Viruses transmitted by sand flies in Italy. Parassitologia. 1991;33(Suppl):513–518. [PubMed] [Google Scholar]
- World Health Organization 1986. Resistance of vectors and reservoirs of disease to pesticides. 10th report of the WHO Expert committee. Vector Biology and Control. Tech Rep Ser, 737.
- World Health Organization 1981. pp. 1–6. Instructions for determining the susceptibility or resistance of blackflies, sand flies and biting midges to insecticides. WHO/VBC/81.810.
- World Health Organization Leishmaniasis. 2007. http://www.who.int/leishmaniasis/en/.
- Yaghoobi-Ershadi MR, Javadian E. Susceptibility status of Phelebotomus papatsi to DDT in the most important focus of zoonotic cutaneous leishmaniasis, Isfahan Province, Iran. Iran J Publ Health. 1995;24:18–24. [Google Scholar]
- Yaghoobi-Ershadi MR, Akhavan AA, Abai MR, Ebrahimi B, Zahraei-Ramazani AR, Vafaei-Nezhad R, Hanafi-Bojd AA, Jafari R. Epidemiological study in a new focus of cutaneous leishmaniasis in the Islamic Republic of Iran. East Mediterr Health J. 2004;10:688–694. [PubMed] [Google Scholar]
- Yaghoobi-Ershadi MR, Akhavan AA, Zahraei-Ramazani AR, Jalali-Zand AR, Piazak N. Bionomics of Phlebotomus papatasi (Diptera: Psychodidae) in an endemic focus of zoonotic cutaneous leishmaniasis in central Iran. J Vector Ecol. 2005;30:115–118. [PubMed] [Google Scholar]
- Yaghoobi-Ershadi MR, Akhavan AA, Zahraei-Ramazani AR, Javadian E, Motavalli-Emami M. Field trial for the control of zoonotic cutaneous leishmaniosis in Badrood, Iran. Ann Saudi Med. 2000;20:386–389. doi: 10.5144/0256-4947.2000.386. [DOI] [PubMed] [Google Scholar]
- Yaghoobi-Ershadi MR, Akhavan AA. Entomological survey of sand flies (Diptera: Psychodidae) in a new focus of zoonotic cutaneous leishmaniosis in Iran. Acta Trop. 1999;15(73):321–326. doi: 10.1016/s0001-706x(99)00038-8. [DOI] [PubMed] [Google Scholar]
- Yaghoobi-Ershadi MR, Hanafi-Bojd AA, Akhavan AA, Zahrai-Ramazani AR, Mohebali M. Epidemiological study in a new focus of cutaneous leishmaniosis due to Leishmania major in Ardestan town, central Iran. Acta Trop. 2001;79:115–121. doi: 10.1016/s0001-706x(01)00085-7. [DOI] [PubMed] [Google Scholar]
- Yaghoobi-Ershadi MR, Hanafi-Bojd AA, Javadian E, Jafari R, Zahraei-Ramazani AR, Mohebali M. A new focus of cutaneous leishmaniasis caused by Leishmania tropica. Saudi Med J. 2002;23:291–294. [PubMed] [Google Scholar]
- Yaghoobi-Ershadi MR, Jafari R, Hanafi-Bojd AA. A new epidemic focus of zoonotic cutaneous leishmaniasis in central Iran. Ann Saudi Med. 2004;24:98–101. doi: 10.5144/0256-4947.2004.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaghoobi-Ershadi MR, Javadian E, Kannani A. Host preference pattern of phlebotomine sand flies of Borkhar rural district, Isfahan province, Iran. Acta Trop. 1995;60:155–158. doi: 10.1016/0001-706x(95)00122-u. [DOI] [PubMed] [Google Scholar]
- Yaghoobi-Ershadi MR, Javadian E, Tahvildare-Bidruni GH. Leishmania major MON-26 isolated from naturally infected Phlebotomus papatasi (Diptera: Psychodidae) in Isfahan Province, Iran. Acta Trop. 1995;59:279–282. doi: 10.1016/0001-706x(95)92834-3. [DOI] [PubMed] [Google Scholar]
- Yaghoobi-Ershadi MR, Javadian E, Tahvildare-Bidruni GH. The isolation of Leishmania major from Phlebotomus (Paraphlebotomus) caucasicus, in Isfahan province, Islamic Republic of Iran. Trans R Soc Trop Med Hyg. 1994;88:518–519. doi: 10.1016/0035-9203(94)90142-2. [DOI] [PubMed] [Google Scholar]
- Yaghoobi-Ershadi MR, Javadian E. Zoonotic cutaneous leishmaniasis to the north of Isfahan. Human infection in 1991. Bull Soc Pathol Exot. 1995;88:42–45. [PubMed] [Google Scholar]
- Yaghoobi-Ershadi MR, Javadian E. Epidemiological study of reservoir hosts in an endemic area of zoonotic cutaneous leishmaniasis in Iran. Bull WHO. 1996;74:587–590. [PMC free article] [PubMed] [Google Scholar]
- Yaghoobi-Ershadi MR, Javadian E. Seasonal variation of Leishmania major infection rates in sand flies from rodent burrows in Isfahan province, Iran. Med Vet Entomol. 1996;10:181–184. doi: 10.1111/j.1365-2915.1996.tb00726.x. [DOI] [PubMed] [Google Scholar]
- Yaghoobi-Ershadi MR, Javadian E. Studies on sand flies in a hyperendemic area of zoonotic cutaneous leishmaniasis in Iran. Indian J Med Res. 1997;105:61–66. [PubMed] [Google Scholar]
- Yaghoobi-Ershadi MR, Moosa-Kazemi SH, Zahraei-Ramazani AR, Jalai-Zand AR, Akhavan AA, Arandain MH, et al. Evaluation of deltamethrin-impregnated bed nets and curtains for control of zoonotic cutaneous leishmaniasis in a hyperendemic area of Iran. Bull Soc Pathol Exot. 2006;99:43–48. doi: 10.3185/pathexo2818. [DOI] [PubMed] [Google Scholar]