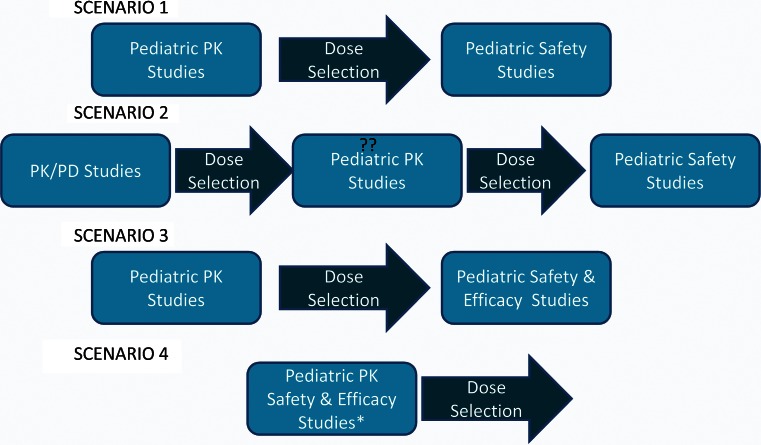
Fig. 1.
The pediatric drug development strategies one may adopt for a specific disease area. Scenarios 1 and 3 are explained in the text. Scenario 2 is also an abbreviated strategy where concentration-response relationships are reconfirmed in a pediatric PK/PD study followed by pediatric safety study. Scenario 4 is a combination of the pediatric PK study and the safety/efficacy study. Double question marks The pediatric PK study can be eliminated with a well-designed pediatric PK/PD study. Asterisk this strategy requires the pivotal study to be a dose ranging study to allow for dose selection at the end of the study