Abstract
Purpose
To visualize the pre-corneal tear film (PCTF) in dry eye patients using ultra-high resolution optical coherence tomography (UHR-OCT).
Methods
A custom built UHR-OCT with ultra-high resolution (~3 µm) was used to image the PCTF at the vertical meridian. Eighteen right eyes of 18 previously diagnosed dry eye patients (9 males and 9 females, age 47.8 ± 20.7 yrs) with aqueous tear deficiency were studied. Images were taken during normal and delayed blinking. The visualized PCTF was measured directly. An indirect calculation was used to obtain the thickness in cases where the PCTF could not be visualized.
Results
During normal blinking, the PCTF was visualized in 5 of 18 eyes (27.8%) with an average PCTF thickness of 5.8 µm (SD 1.3 µm). During delayed blinking, the PCTF was visualized in 11 eyes (61.1%) with a significantly increased average thickness of 7.3 µm (SD 0.9 µm, P <.05). The percent increase of the visualized PCTF thickness was higher during delayed blinking compared to normal blinking (chi-squared test, P < .05). The averaged PCTF was 4.4 µm during normal blinking, and the PCTF thickness was significantly increased to 6.6 µm (SD 2.9 µm, P < .05) during delayed blinking.
Conclusions
We reported the first visualization of PCTF in vivo in dry eye patients with UHR-OCT. PCTF can be directly visualized in some eyes during both normal and delayed blinking. PCTF appeared thicker during delayed blinking compared to normal blinking.
Keywords: tear film, optical coherence tomography, dry eye
Introduction
The pre-corneal tear film (PCTF) protects the ocular surface from damage. During blinking, tears from the tear menisci around the upper and lower eyelids form the tear film with the aid of blinking.1,2 The tear film renews after every blink and thins during eye opening, and it often dries out at some locations on the ocular surface, resulting in dry spots.1,3–7 In normal eyes, the coverage of the tear film on the ocular surface lasts long enough before the next blink. However, in dry eyes, due to compromised tear quality and quantity,8 the tear film breaks within a short period, resulting in damage to the ocular surface and dry eye symptoms.8–10 Patients with aqueous tear deficiency (ATD) also have a reduced tear meniscus volume.11 If the tear film is thin, it may not be possible to visualize and measure its thickness using an optical section with slit-lamp biomicroscopy. It is unknown whether the tear film in dry eye patients can be directly visualized and measured with optical coherence tomography (OCT). Optical coherence tomography is a non-contact and non-invasive imaging modality that is able to image the tear film without disturbing the tear system.12,13 Ultra-high resolution optical coherence tomography (UHR-OCT) has been used to image the tear film underneath contact lenses12 and allows for visualization of the tear film in healthy subjects.13 The goal of the present study was to determine whether the pre-corneal tear film (PCTF) can be directly visualized using UHR-OCT in aqueous tear deficiency (ATD) dry eye patients.
Methods and Subjects
This study was approved by the research review board of the University of Miami and was conducted in accordance with the tenets of the Declaration of Helsinki. Informed consent was obtained from each participant prior to enrollment in the study. Eighteen consecutive clinically diagnosed ATD dry eye patients (9 women and 9 men, mean ± standard deviation age: 47.8 ± 20.7 years) were recruited for the study. Dry eye was diagnosed based on the results of a questionnaire that reported dry eye symptoms and signs, including the Schirmer test ≤5 mm/5 min, Tear break up time (TBUT) ≤10 s and the absence of Meibomian gland or eyelid diseases, as observed through a slit-lamp examination.14,15 The Schirmer test was performed with anesthesia. A test strip was placed inside the conjunctival sac, and the eye was closed for 5 minutes. TBUT was recorded after the instillation of fluorescein through a standard slit-lamp with cobalt blue light and a yellow filter. The time until the first appearance of dry spots after blinking was recorded. These diagnostic tests were conducted during the previous clinic visits. Eligible patients were enrolled in this study. Those with a history of contact lens use, ophthalmic surgery, and any ocular or systemic disease other than dry eye were excluded.
Ultra-high resolution spectral domain OCT
To visualize the central PCTF, a custom-built, high-speed UHR-OCT (~3 µm) instrument was used for this study.12,13 Briefly, the light source was a three-module superluminescent diode (Broadlighter, T840-HP, Superlumdiodes Ltd., Moscow, Russia) with a center wavelength of 840 nm and a full width at half of the maximum bandwidth of 100 nm. The sample light was delivered to a light delivery system with a telecentric design that consisted of an X-Y galvanometer scanner. The power of the incident light delivered into the anterior segment was lowered to 750 µW to ensure the safety of the eye. The scan width was set to 12 mm with a scan depth of 3 mm, and the speed of the system was set to 24K A-scans per second.
Experimental procedure and image processing
The study was conducted in an examination room with the same lab settings as described in previous studies.7,16 Central air conditioning maintained the temperature within a range from 15 to 25°C and the relative humidity at 30 to 50%. Only ambient room light was used to maintain dim lighting while each subject was imaged. The right eye of each subject was tested. OCT imaging was performed at least 2 hours after the instillation of any drops. The central OCT beam, which could be seen on the OCT monitor, was set on the corneal apex where a specular reflection was normally detected. Continuous OCT scanning was performed at the vertical meridian while the subjects were asked to look at an external target and blink normally (normal blinking). After that, a similar OCT scan was taken when the subjects were asked to delay each blink as long as possible (delayed blinking). Only the frames acquired immediately after each blink during normal and delayed blinking were analyzed to determine the PCTF thickness. If the PCTF was visualized, custom software was used to detect its anterior and posterior surfaces and to calculate the thickness directly (Fig. 1). If the PCTF was not visualized, the central PCTF thickness was obtained indirectly.7,12 First, the thickness of the central cornea plus the PCTF was measured and designated. After the instillation of one drop of artificial tears, the true central corneal thickness was obtained. Consequently, the central PCTF thickness was calculated as the thickness of the central cornea plus the PCTF, subtracting the true central corneal thickness.
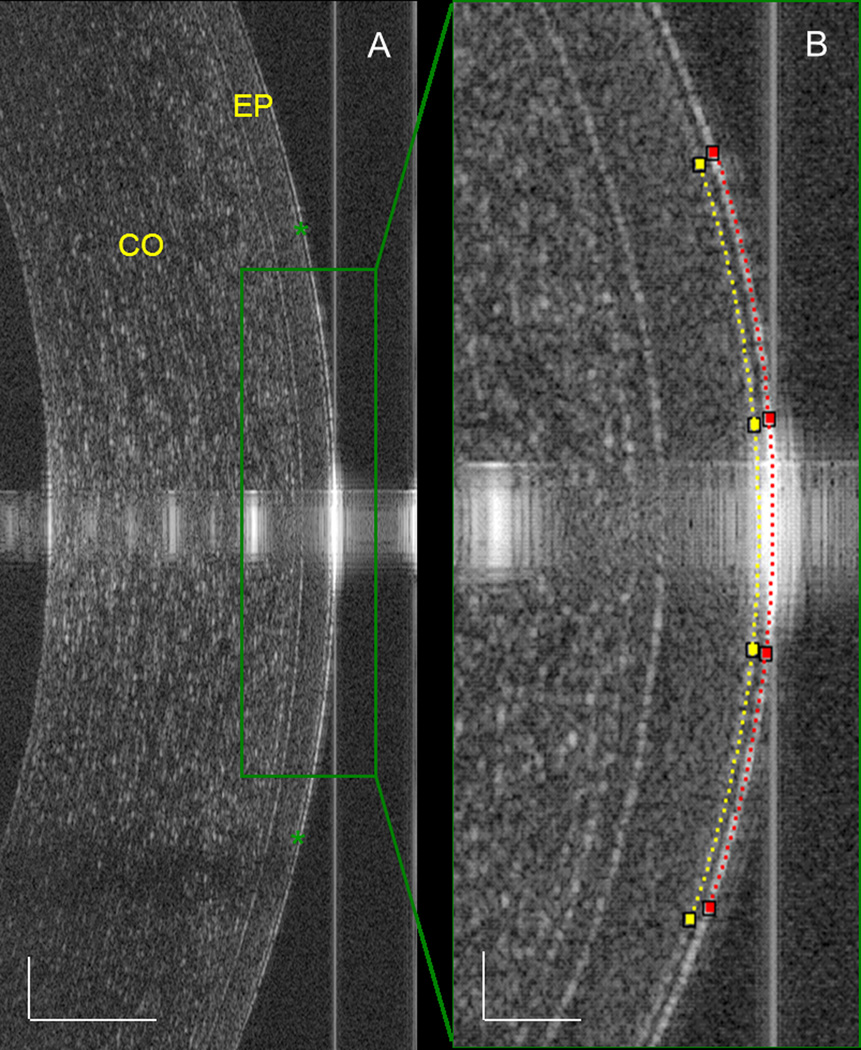
Figure 1. OCT of the PCTF in a dry eye patient during delayed blinking.
(A) Overview of the cornea showing the PCTF on the ocular surface (green asterisk). Bars = 250 µm. (B) For the highly magnified image of the central cornea, custom software was used to detect the anterior (red dotted line) and posterior surface (yellow dotted line) of the PCTF. The thickness of the central PCTF was calculated directly from the images. Bars = 50 µm. CO, cornea; EP, epithelium of the cornea.
Data analysis
Data analysis was conducted using the Statistical Package for the Social Sciences (SPSS, version 17.0 for Windows XP; SPSS Inc., Chicago, IL). Descriptive data were calculated as the means ± standard deviations. The chi-square test was used to determine if there were differences in the frequency of PCTF visualization between normal and delayed blinking. The Mann-Whitney U test was used to determine if there were any differences in the thickness of the PCTF between normal and delayed blinking. P < .05 was considered significant.
Results
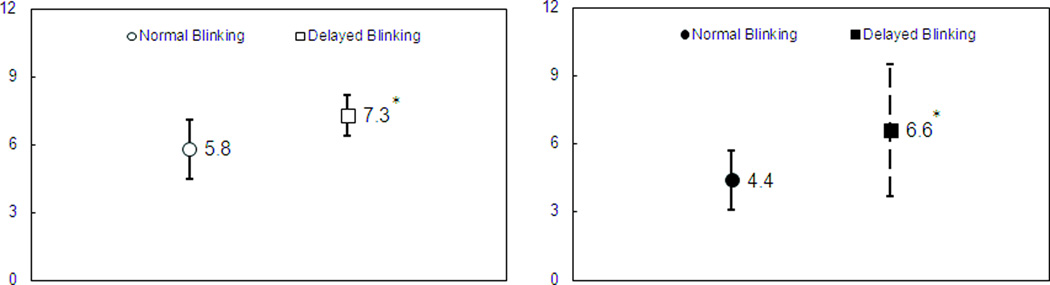
During normal blinking, the PCTF was visualized in only 5 of 18 ATD eyes (27.8%, Table 1). In contrast, the PCTF was visualized in 11 eyes (61.1%) after delayed blinking, which was significantly more than after normal blinking (chi-square test, P < .05; Table 1). The thickness of the directly visualized PCTF after normal blinking was 5.8 ± 1.3 µm (n = 5, Table 2, Fig. 2), and during delayed blinking it increased to 7.3 ± 0.9 µm (n = 11, P < .05, Table 2, Fig. 3). The PCTF thickness calculated as the average of the directly visualized thickness and the indirectly calculated thickness was 4.4 ± 1.3 µm during normal blinking (Table 2). For delayed blinking, the thickness was 6.6 ± 2.9, which was significantly thicker than for normal blinking (4.4 ± 1.3 µm, n = 18, P < .05, Table 2).
Table 1.
Frequency of the visualization of the PCTF during normal and delayed blinking.
| Blinking | PCTF* |
|
|---|---|---|
| Visualized | Not visualized | |
| Normal | 5 (27.8%) | 13 (72.2%) |
| Delayed | 11 (61.1%) | 7 (38.9%) |
The percentage of the visualized PCTF was higher after delayed blinking compared to normal blinking (chi-square test, P < .05).
Table 2.
Thickness of the PCTF during normal and delayed blinking.
| Directly visualized thickness (µm) |
Indirectly calculated thickness (µm) |
Average thickness* (µm) |
|
|---|---|---|---|
| Normal Blink | |||
| Mean ± SD (eyes) | 5.8 ± 1.3 (5) † | 3.9 ± 0.9 (13) † | 4.4 ± 1.3 (18) † |
| Range | 4.3 ~ 7.4 | 2.0 ~ 7.2 | 2.0 ~ 7.4 |
| Delayed Blink | |||
| Mean ± SD (eyes) | 7.3 ± 0.9‡ (11) † | 5.6 ± 4.5 (7) † | 6.6 ± 2.9‡ (18) † |
| Range | 5.6 ~ 8.3 | 0.4 ~ 12.0 | 0.4 ~ 12.0 |
Average thickness was calculated as the mean value of the directly visualized PCTF and the indirectly calculated PCTF thicknesses.
number of eyes,
P < .05 when compared to normal blinking.
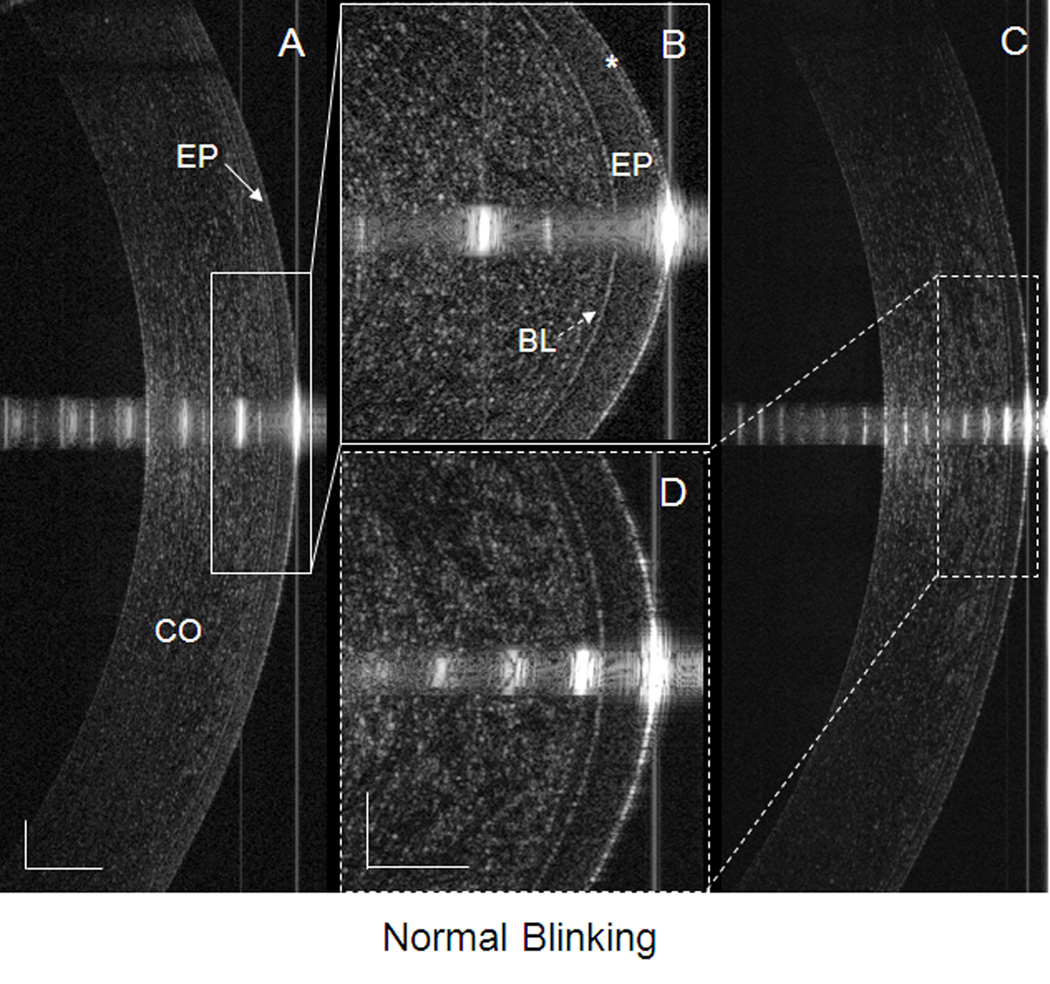
Figure 2. PCTF after a normal blink in a dry eye patient.
Images were taken by UHR-OCT at the center of the cornea immediately after a blink. (A) The PCTF was visualized and (B) can be readily observed (asterisk). The thickness of the visualized PCTF was 5.8 µm. (C) The PCTF was not visualized, even after enlargement of the image (D). CO, cornea; EP, epithelium of the cornea (solid arrow in A); BL, Bowman’s layer of the cornea (dotted arrow in B). The bars denote 250 µm for two of the images (A and C) and 100 µm for the other two images (B and D).
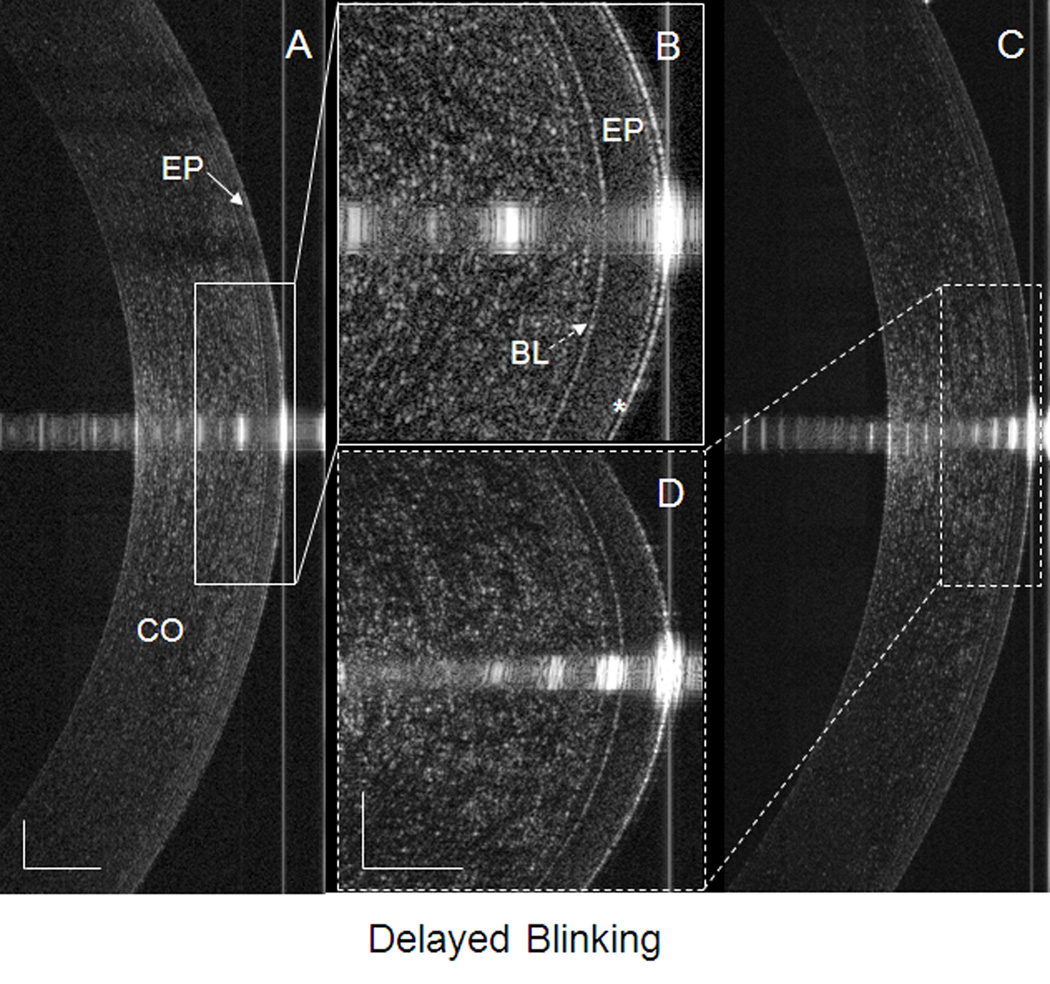
Figure 3. PCTF after delayed blinking in a dry eye patient.
Images were taken by UHR-OCT at the center of the cornea immediately after a delayed blink. (A) The PCTF was visualized and (B) can be readily observed (asterisk). The thickness of the visualized PCTF was 7.3 µm. (C) The PCTF was not visualized, even after enlargement of the image (D). CO, cornea; EP, epithelium of the cornea (solid arrow in A); BL, Bowman’s layer of the cornea (dotted arrow in B). The bars denote 250 µm for two of the images (A and C) and 100 µm for the other two images (B and D).
Discussion
The eye requires tears with high quality and quantity to maintain the integrity of the ocular surface by forming a thin film like tear layer.1,8 The tear film provides a smooth surface that is important for sharp vision during the eye-opening period.17 It also provides lubrication to reduce possible damage from shear forces between the eyelid and the ocular surface during blinking.18,19 Studying tear film morphometry may provide a better understanding mechanisms involved in dry eye.20 The thickness of the tear film has long been studied using different methods.21–26 The results have shown that the tear film continuously changes during the blinking cycle.7,19,27–29 The thickness ranges from several micrometers21,22,25 to 40 micrometers26 if tearing or the installation of artificial tears occurs. Using interferometric methods, the normal tear film thickness in healthy eyes was reported to be approximately 3 µm.25 As another non-invasive method to evaluate the PCTF, UHR-OCT was used to measure the dynamic changes in the tear film on contact lenses by Chen et al.12 After the instillation of artificial tears, the tear film underneath the contact lens was visualized using UHR-OCT.13 The results suggested that UHR-OCT with 3-µm resolution may be used to visualize the tear film. Based on these previous studies,12,13 the present study demonstrated that UHR-OCT could be used to visualize the tear film in dry eye patients, especially during delayed blinking.
In the present study, the PCTF was visualized in 5 dry eyes during normal blinking and 11 dry eyes during delayed blinking. This study represents the first report of the visualization of the PCTF in dry eye patients using UHR-OCT, and we found that the visualization frequency increased during delayed blinking compared to normal blinking. Based on measurements taken directly from the UHR-OCT images, the PCTF was thicker during delayed blinking than during normal blinking. The mean thickness of the directly visualized PCTF during normal blinking was 5.8 µm, and the lowest individual value was 4.3 µm. These values were well above the ~ 3-µm limit of axial resolution for UHR-OCT.12 Based on the resolution, any PCTF that could be measured was greater than 3 µm in thickness.
The PCTF appears to rely on the tear meniscus volume and blinking, which spreads the tears onto the ocular surface.1 Due to the reduced tear secretion resulting in lower tear volume in the tear menisci,11 the averaged PCTF thickness of dry eye patients may be thinner than the previously reported PCTF thickness in normal eyes. Interestingly, we did not find extremely thinned tear films in the dry eye patients, and the tear film in some of the dry eyes was visualized. This may indicate that the tear film of several micrometers may be formed, even when the tear volume in the tear meniscus is lower. In addition to other factors, such as friction with the lid, the velocity and smoothness of the lid movement and the epithelial surface smoothness, the quality of the tear film may play a large role in the development of dry eye symptoms and signs. Wang et al. reported that the average thickness of the PCTF was approximately 3 µm in healthy eyes.3,30 Interferometric tear film thickness measurement by Hosaka et al.31 showed that the tear film was approximately 2.0 µm thick in dry eye patients. The differences between the pervious results and the present study were mainly due to the different periods of image acquisition. In the present study with dry eye patients, the images were acquired immediately after blinking. As documented in several previous studies, the thickness of the PCTF varies over time; it is thicker immediately after blinking and thinner during open-eye periods.7,19,27–29 The PCTF was thickest immediately after each blink. In our previous study, the measurements were taken midway between blinks.30 In the study by Hosaka and associates,31 the authors measured the tear film thickness 5 seconds after a complete blink, and most dry eye patients in their study were Sjögren syndrome (SS) patients who had a more severe dry eye condition than the patients in the current study.
Due to reflex tearing, the balance of tear secretion and drainage in normal eyes has been found to be altered during delayed blinking.4 During delayed blinking, the tear menisci increased significantly.7,11 Palakuru et al.7 found no significant difference in the PCTF thickness between normal and delayed blinks in normal eyes. However, the resolution of the time-domain OCT that they used was insufficient to detect the small changes. In our dry eye patients, the average PCTF thickness was higher immediately after delayed blinking than after normal blinking. The reason may be that the enrolled patients had mild dry eye because TBUT<10 seconds was used as an inclusion criteria for dry eye in this study. The increase of PCTF during delayed blinking may also indicate that patients with dry eye may have some ability to produce tears in response to a challenging situation such as prolonged eye opening. The increase in the tear volume during delayed blinking in dry eye patients was demonstrated in our previous study.11 The relationship between PCTF thickness and tear volume was also established in our previous studies with delayed blinking7 and the instillation of artificial tears into normal eyes.16 Although no tear meniscus was imaged in the present study, the increase of the tear meniscus volumes in these dry eye patients may have resulted in an increase of the PCTF thickness visualized with UHR-OCT. However, the capability of tearing may be compromised, as Farris and associates32 found that dry eye patients were deficient in both basal and reflex tears. A small increase of the tears might give rise to a visible increase of the PCTF thickness due to the micrometer-thin PCTF itself. Interestingly, based on the increase of PCTF thickness observed during the delayed blinking studies, one may suggest that the delayed blinking frequency may be beneficial to dry eye patients. This viewpoint should be investigated in future studies.
UHR-OCT may have a greater range than other tear film imaging methods, such as tear interferometry,25 especially for the direct visualization of the tear film. The limitation of its poor detection rate may be improved by further improvement of axial resolution. In the present study, we sought to visualize the tear film in dry eye patients. There are some limitations in the present study. First, the thicknesses of PCTFs that could not been visualized were measured indirectly, and this might have resulted in some error. However, significant increases of PCTF were still found after delayed blinking in the present study. Second, the tear film thickness was studied in dry eye patients only, and the tear meniscus dimensions were not measured. However, in our previous study, the tear menisci were reported to be lower in dry eye patients. Better knowledge of the relationship between the tear menisci and the tear film in dry eye patients might lead to a better understanding of dry eye, and future studies will be needed to correlate these measurements. Third, the sample size of dry eye patients used to compare the effects of normal and delayed blinking was relatively small. Even so, the PCTF thickness after delayed blinking was greater than after normal blinking. Fourth, the failure to visualize the PCTF in some eyes was mainly due to the 3-µm resolution limit for the current generation of OCT instruments. Further improvement of OCT resolution is underway and may soon be applied to studies of tear film dynamics.
In summary, this was the first study to use UHR-OCT for the in vivo visualization of the PCTF in dry eye patients. The PCTF was directly visualized on many eyes so that the thickness could be directly calculated. For other eyes, the thickness could be determined indirectly. For dry eye patients, delayed blinking increased the thickness of the PCTF.
Figure 4. Thickness of the visualized PCTF and the average PCTF thickness during normal and delayed blinking.
(A) Thickness of the visualized PCTF during normal and delayed blinking. (B) Average thickness of the PCTF during normal and delayed blinking. (*) indicates that the thicknesses of the PCTF were higher during delayed blinking compared to normal blinking (P <.05).
Acknowledgement
This study was supported in part by research grants from Allergan, the Wallace H. Coulter Center, the NIH Center Grant P30 EY014801 and Research to Prevent Blindness (RPB). We wish to thank Dr. Britt Bromberg of Xenofile Editing for providing editing services for this manuscript.
Commercial relationship: Jianhua Wang is a recipient of the research grant from Allergan, Inc..
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: The authors (LC, JW, VP, MS, YY and MW) have no proprietary interest in any materials or methods described within this article.
References
- 1.Doane MG. Blinking and the mechanics of the lacrimal drainage system. Ophthalmology. 1981;88:844–851. doi: 10.1016/s0161-6420(81)34940-9. [DOI] [PubMed] [Google Scholar]
- 2.Tsubota K, Nakamori K. Effects of ocular surface area and blink rate on tear dynamics. Arch Ophthalmol. 1995;113:155–158. doi: 10.1001/archopht.1995.01100020037025. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Aquavella J, Palakuru J, Chung S, Feng C. Relationships between central tear film thickness and tear menisci of the upper and lower eyelids. Invest Ophthalmol Vis Sci. 2006;47:4349–4355. doi: 10.1167/iovs.05-1654. [DOI] [PubMed] [Google Scholar]
- 4.Sahlin S, Laurell CG, Chen E, Philipson B. Lacrimal drainage capacity, age and blink rate. Orbit. 1998;17:155–159. doi: 10.1076/orbi.17.3.155.2757. [DOI] [PubMed] [Google Scholar]
- 5.Sahlin S, Chen E. Gravity, blink rate, and lacrimal drainage capacity. Am J Ophthalmol. 1997;124:758–764. doi: 10.1016/s0002-9394(14)71692-7. [DOI] [PubMed] [Google Scholar]
- 6.Holly FJ. Formation and stability of the tear film. Int Ophthalmol Clin. 1973;13:73–96. [PubMed] [Google Scholar]
- 7.Palakuru JR, Wang J, Aquavella JV. Effect of blinking on tear dynamics. Invest Ophthalmol Vis Sci. 2007;48:3032–3037. doi: 10.1167/iovs.06-1507. [DOI] [PubMed] [Google Scholar]
- 8.Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:108–152. doi: 10.1016/s1542-0124(12)70083-6. [DOI] [PubMed] [Google Scholar]
- 9.Bron Anthony J. Diagnosis of dry eye. Surv Ophthalmol. 2001;45(Suppl 2):s221–s226. doi: 10.1016/s0039-6257(00)00201-0. [DOI] [PubMed] [Google Scholar]
- 10.Pflugfelder SC, Tseng SC, Sanabria O, et al. Evaluation of subjective assessments and objective diagnostic tests for diagnosing tear-film disorders known to cause ocular irritation. Cornea. 1998;17:38–56. doi: 10.1097/00003226-199801000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Yuan Y, Wang J, Chen Q, et al. Reduced tear meniscus dynamics in dry eye patients with aqueous tear deficiency. Am J Ophthalmol. 2010;149:932–938. doi: 10.1016/j.ajo.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Q, Wang J, Tao A, et al. Ultrahigh-resolution measurement by optical coherence tomography of dynamic tear film changes on contact lenses. Invest Ophthalmol Vis Sci. 2010;51:1988–1993. doi: 10.1167/iovs.09-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Jiao S, Ruggeri M, Shousha MA, Chen Q. In situ visualization of tears on contact lens using ultra high resolution optical coherence tomography. Eye Contact Lens. 2009;35:44–49. doi: 10.1097/ICL.0b013e31819579f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin PY, Tsai SY, Cheng CY, et al. Prevalence of dry eye among an elderly Chinese population in Taiwan: the Shihpai Eye Study. Ophthalmology. 2003;110:1096–1101. doi: 10.1016/S0161-6420(03)00262-8. [DOI] [PubMed] [Google Scholar]
- 15.Schein OD, Tielsch JM, Munoz B, Bandeen-Roche K, West S. Relation between signs and symptoms of dry eye in the elderly. A population-based perspective. Ophthalmology. 1997;104:1395–1401. doi: 10.1016/s0161-6420(97)30125-0. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Aquavella J, Palakuru J, Chung S. Repeated measurements of dynamic tear distribution on the ocular surface after instillation of artificial tears. Invest Ophthalmol Vis Sci. 2006;47:3325–3329. doi: 10.1167/iovs.06-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rolando M, Zierhut M. The ocular surface and tear film and their dysfunction in dry eye disease. Surv Ophthalmol. 2001;45(Suppl 2):S203–S210. doi: 10.1016/s0039-6257(00)00203-4. [DOI] [PubMed] [Google Scholar]
- 18.Doane MG. Interactions of eyelids and tears in corneal wetting and the dynamics of the normal human eyeblink. Am J Ophthalmol. 1980;89:507–516. doi: 10.1016/0002-9394(80)90058-6. [DOI] [PubMed] [Google Scholar]
- 19.Lemp MA. Advances in understanding and managing dry eye disease. Am J Ophthalmol. 2008;146:350–356. doi: 10.1016/j.ajo.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Murube J. Tear osmolarity. Ocul Surf. 2006;4:62–73. doi: 10.1016/s1542-0124(12)70028-9. [DOI] [PubMed] [Google Scholar]
- 21.MISHIMA S. SOME PHYSIOLOGICAL ASPECTS OF THE PRECORNEAL TEAR FILM. Arch Ophthalmol. 1965;73:233–241. doi: 10.1001/archopht.1965.00970030235017. [DOI] [PubMed] [Google Scholar]
- 22.Benedetto DA, Shah DO, Kaufman HE. The instilled fluid dynamics and surface chemistry of polymers in the preocular tear film. Invest Ophthalmol. 1975;14:887–902. [PubMed] [Google Scholar]
- 23.Ehlers N. The thickness of the precorneal tear film. Acta Ophthalmol. 1965;81:92–100. [Google Scholar]
- 24.Danjo Y, Nakamura M, Hamano T. Measurement of precorneal tear film thickness with a non-contact optical interferometry film thickness measurement system. Jpn J Ophthalmol. 1994;38:260–266. [Google Scholar]
- 25.King-Smith PE, Fink BA, Fogt N, et al. The thickness of the human precorneal tear film: evidence from reflection spectra. Invest Ophthalmol Vis Sci. 2000;41:3348–3359. [PubMed] [Google Scholar]
- 26.Prydal JI, Artal P, Woon H, Campbell FW. Study of human precorneal tear film thickness and structure using laser interferometry. Invest Ophthalmol Vis Sci. 1992;33:2006–2011. [PubMed] [Google Scholar]
- 27.Benedetto DA, Clinch TE, Laibson PR. In vivo observation of tear dynamics using fluorophotometry. Arch Ophthalmol. 1984;102:410–412. doi: 10.1001/archopht.1984.01040030328030. [DOI] [PubMed] [Google Scholar]
- 28.Nichols JJ, Mitchell GL, King-Smith PE. Thinning rate of the precorneal and prelens tear films. Invest Ophthalmol Vis Sci. 2005;46:2353–2361. doi: 10.1167/iovs.05-0094. [DOI] [PubMed] [Google Scholar]
- 29.Montes-Mico R, Alio JL, Munoz G, Charman WN. Temporal changes in optical quality of air-tear film interface at anterior cornea after blink. Invest Ophthalmol Vis Sci. 2004;45:1752–1757. doi: 10.1167/iovs.03-0839. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Fonn D, Simpson TL, Jones L. Precorneal and pre- and postlens tear film thickness measured indirectly with optical coherence tomography. Invest Ophthalmol Vis Sci. 2003;44:2524–2528. doi: 10.1167/iovs.02-0731. [DOI] [PubMed] [Google Scholar]
- 31.Hosaka E, Kawamorita T, Ogasawara Y, et al. Interferometry in the evaluation of precorneal tear film thickness in dry eye. Am J Ophthalmol. 2011;151:18–23. doi: 10.1016/j.ajo.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 32.Farris RL, Stuchell RN, Mandel ID. Basal and reflex human tear analysis. I. Physical measurements: osmolarity, basal volumes, and reflex flow rate. Ophthalmology. 1981;88:852–857. doi: 10.1016/s0161-6420(81)34939-2. [DOI] [PubMed] [Google Scholar]