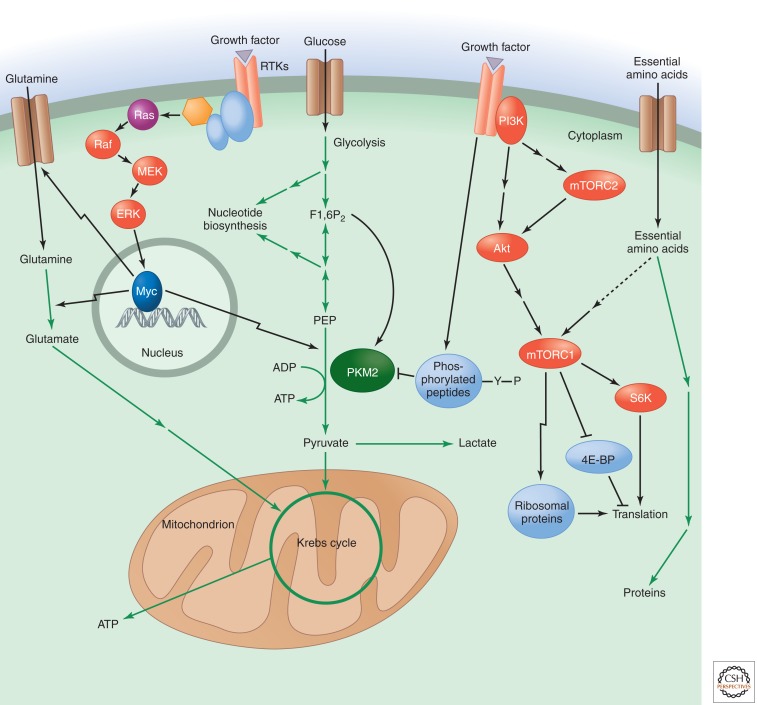
Figure 4.
Pyruvate kinase as a glycolytic switch, and mTOR and Myc regulate amino acid uptake and metabolism. Pyruvate kinase catalyzes the late step of glycolysis that converts phosphoenolpyruvate (PEP) to pyruvate, with the concomitant generation of ATP. The M2 isoform of pyruvate kinase (PKM2) is specific to proliferating cells. Its activity is allosterically activated by the upstream glycolytic intermediate fructose 1,6-bisphosphate (F1,6P2). Tyrosine kinase signaling downstream from growth factor receptors causes the release of F1,6P2 from PKM2, which results in decreased pyruvate kinase enzyme activity. This enzymatic inhibition can promote the redistribution of upstream glycolytic intermediates into anabolic pathways like nucleotide biosynthesis. The transcription factor Myc positively regulates the expression of PKM2 versus the constitutively active PKM1 isoform by up-regulating several RNA splicing factors. Glutamine uptake and metabolism are also regulated by the Myc transcription factor, which can be activated downstream from growth factor signaling pathways, such as those involving Ras. Myc positively regulates the expression of glutamine transporters as well as the enzyme glutaminase. Glutamate, the product of glutaminase activity, can be further metabolized to α-ketoglutarate to supply intermediates for the mitochondrial Krebs cycle. Another major regulator of amino acid metabolism is mTOR complex 1 (mTORC1), which can be activated downstream from PI3K/Akt signaling as well as through the sensing of essential amino acids. mTORC1 positively regulates protein synthesis in response to these inputs by activating S6 kinase (S6K), inhibiting eukaryotic initiation factor 4E binding protein (4E-BP), and promoting the expression of ribosomal proteins. A distinct mTOR complex, mTORC2, lies upstream of Akt and is positively regulated by PI3K signaling.