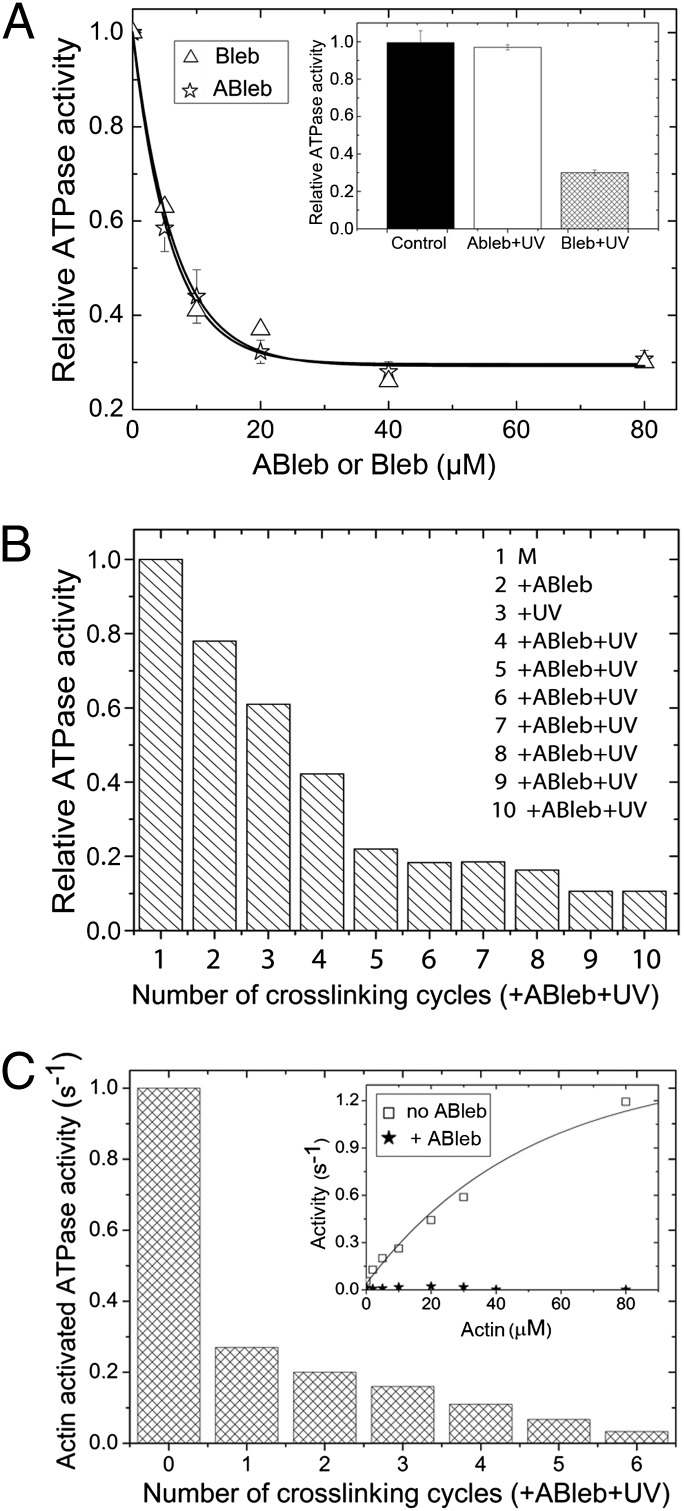
Fig. 3.
Myosin ATPase inhibition by azidoblebbistatin in the absence and presence of actin. (A) Inhibition of the basal (actin-free) ATPase activity of DdMd in the absence of irradiation. Relative ATPase activities of 2 μM DdMd at increasing concentrations of blebbistatin (Bleb) or azidoblebbistatin (ABleb) are shown. Hyperbolic fits to the datasets yielded IC50 values of 6.4 ± 0.9 μM and 5.2 ± 0.3 μM for blebbistatin and azidoblebbistatin; the maximal extent of inhibition was 71% and 70%, respectively. (Inset) Irradiation of azidoblebbistatin before adding it to the protein completely abolished its inhibitory effect, whereas the same treatment did not affect blebbistatin inhibition. (B) Relative basal DdMd ATPase activities measured after a series of crosslinking rounds with azidoblebbistatin. Eight μM DdMd (M, column 1) was treated with 10 μM azidoblebbistatin (column 2), then crosslinked with UV light (column 3). The complex was further sequentially treated with 10-μM additions of azidoblebbistatin and UV irradiation (columns 4–10). In the reaction series, practically all myosin molecules were crosslinked, as indicated by the saturation in the inhibition of the ATPase activity. Importantly, ATPase activities were only affected by the covalently crosslinked azidoblebbistatin, because the azidoblebbistatin in solution was degradated completely. (C) Actin-activated ATPase activities of DdMd (in 50 μM actin) upon sequential azidoblebbistatin crosslinking cycles. Sequential 10 μM azidoblebbistatin addition plus UV irradiation cycles were performed on 4 μM DdMd. (Inset) The actin titration profile of untreated (□) and completely azidoblebbistatin-crosslinked (★) DdMd. Actin activated the untreated DdMd ATPase activity to a maximal extent of 1.4 ± 0.06 s−1 with half-maximal activation at 50 μM actin. In contrast, the ATPase activity of crosslinked DdMd remained unaffected by the presence of actin.