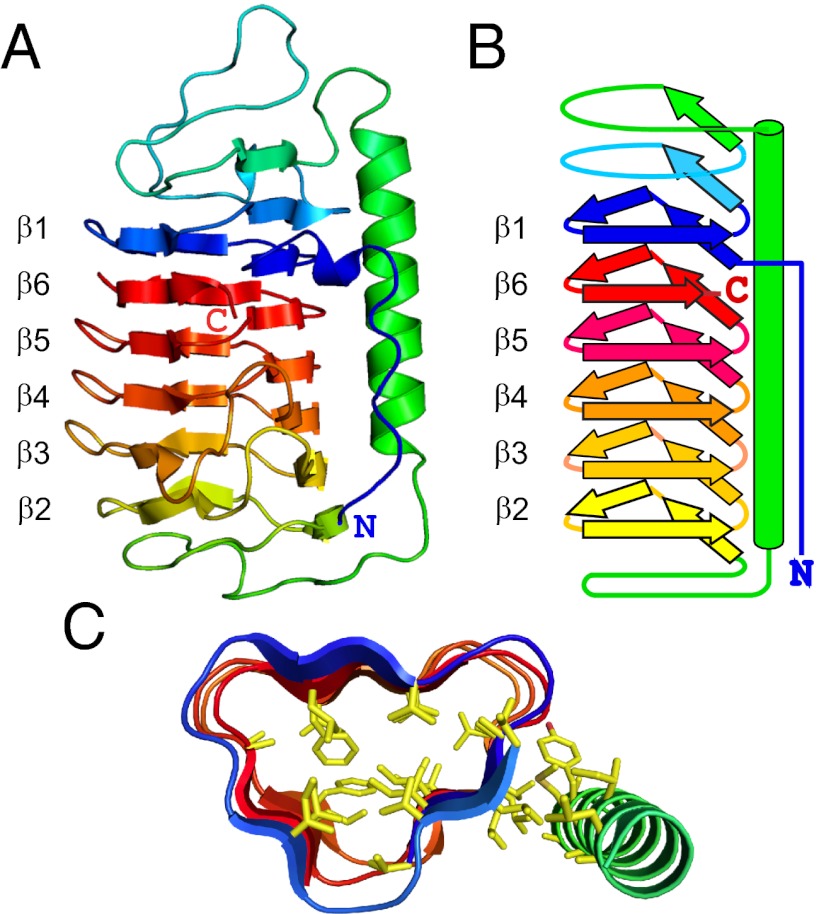
Fig. 1.
Structure of TisAFP6. (A) Ribbon presentation of TisAFP6 structure in spectral color gradation from blue (N terminus) to red (C terminus). The six loops of the right-handed β-helix are numbered β1 to β6 to emphasize their irregular order, β1–β6–β5–β4–β3–β2, in the helix due to the unique positioning of the C-terminal loop, β6, adjacent to the N-terminal loop, β1. The 22-residue-long α-helix lying parallel to the β-helix axis is colored green. (B) Schematic representation of the structure of TisAFP6 illustrating how each loop comprises three short β-strands twisted at an angle of 60° to form the corners of the β-helix. The structure is colored as in A. (C) Triangular cross-section of a central portion of TisAFP6 comprising loops β1–β6–β5–β4 showing the packing of hydrophobic residues (yellow) in the core and between the α- and β-helices. Amino acid sequence of TisAFP6 is shown in Fig. 5.