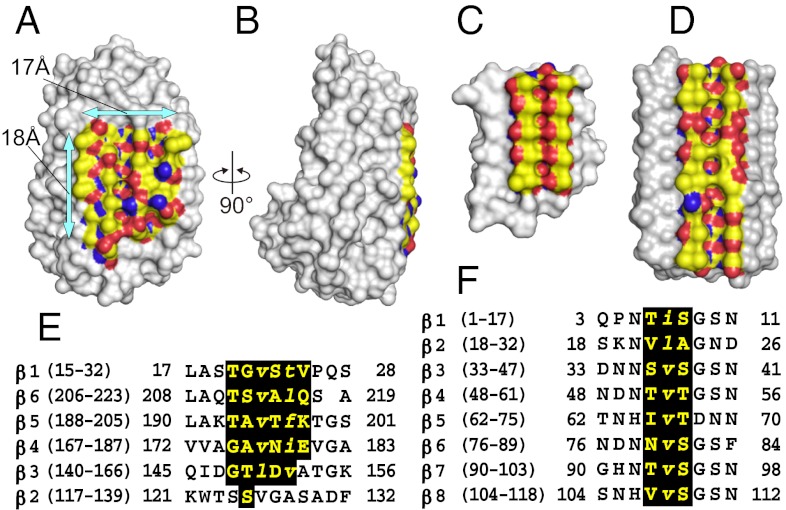
Fig. 3.
Comparison of the TisAFP6 IBS with those of sbwAFP and LpAFP. (A) Front view of the b-face of TisAFP6 showing the putative IBS bound by arrows. Aliphatic side chains, hydroxyl group, and nitrogen atoms are colored yellow, red, and blue, respectively. (B) IBS viewed side on to show its flatness and the pear-shaped other side of TisAFP6. (C) Space-filling view of sbwAFP showing the regularity and flatness of its IBS resulting from the alignment of six TXT ice-binding motifs. Color use is the same as in A. (D) IBS of LpAFP from the grass Lolium perenne. The AFP is a left-handed β-roll with eight 14- to 15-residue coils. (E) Alignment of the β-strand sequences of β1–β6 loops of TisAFP6 that comprise the IBS. Large and small letters indicate the residues having their side chains directed toward the outside and inside, respectively. (F) Alignment of the selected sequences of β1–β8 loops of LpAFP to highlight the residues constructing its putative IBS.