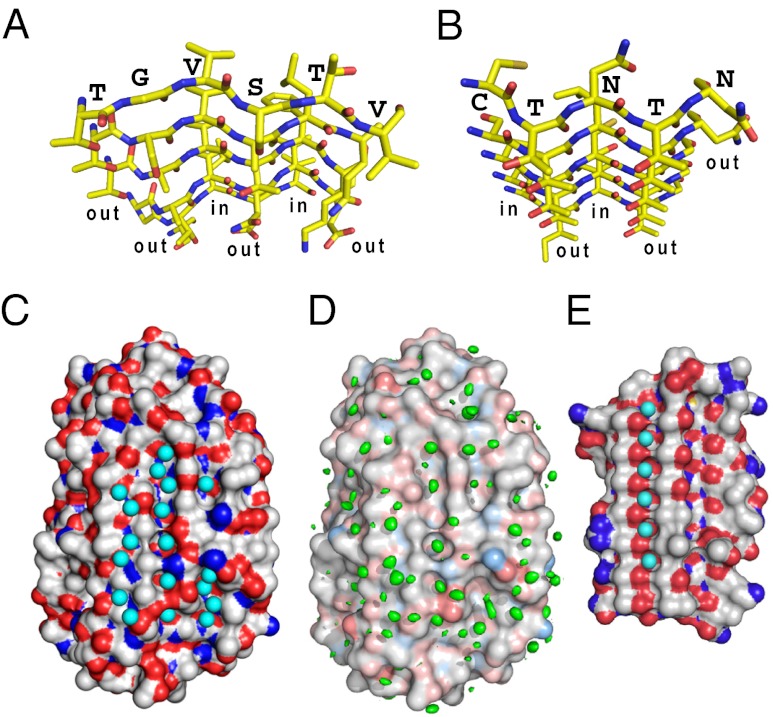
Fig. 4.
Comparison of IBS and surface waters between TisAFP6 and sbwAFP. (A) N- to C-terminal view down the six parallel β-strands constructing the IBS of TisAFP6 characterized by a repetitive out-out-in-out-in-out side-chain motif. Residue assignments are only indicated for the β1-strand. O atoms are red and N atoms are blue. (B) N- to C-terminal view down the six parallel β-strands constructing the IBS of sbwAFP characterized by a repetitive out-out-in-out-in-out side-chain motif. Residue assignments are only indicated for the β1-strand. (C) Positions of 56 waters contacting the crystal structure of TisAFP6; 18 are located in three troughs created between the linearly aligned first, second, fourth, and sixth residues of the IBS β-strands. (D) Positions of waters determined by MD analysis. Structure shown is that at the start of the 12-ns MD simulation performed at 273 K. A total of 604 frames captured during the 12-ns MD simulation were aligned to the initial structure via the α-carbon of each amino acid residue. The volume map tool of VMD was used to calculate the density for just the oxygen atoms of the water molecules. The resulting water density map is colored green and is contoured at 0.9 σ. (E) Regularly aligned and immobilized waters located in a trough on the IBS of sbwAFP.