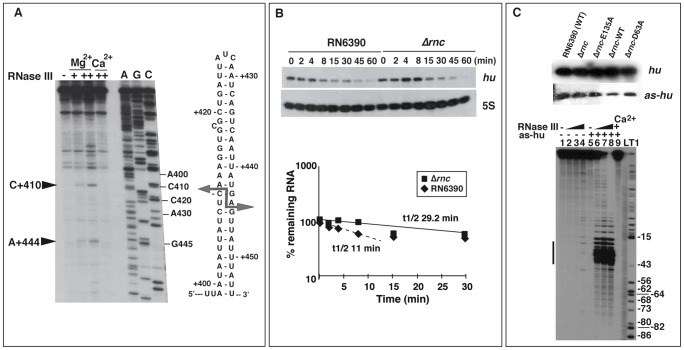
Figure 7. Effect of RNase III on mRNA turnover.
(A) RNase III cleavage assays on in vitro transcribed secY mRNA. The cleavages were assigned after primer extension using 5′ end labeled oligonucleotide 292 (Table S8). RNase III cleavage reactions were done in the absence (−) and in the presence of RNase III (+, 0.33 µM; ++ 0.65 µM) in a buffer containing Mg2+ or Ca2+. Lanes C, G, A: Sequencing reactions. The arrows denote the RNase III-induced cleavages, which are reported on the secondary structure of the RNase III binding site located in the coding sequence of secY mRNA. (B) Analysis of hu mRNA expression in RN6390 and the isogenic Δrnc mutant strain. Upper panel: measurements of the half-life of hu mRNA by monitoring mRNA levels after rifampicin treatment as a function of time (min). A strand specific labeled riboprobe was used to detect hu mRNA. The riboprobe was transcribed in vitro with T7 RNA polymerase using a PCR template amplified with the oligonucleotides 370 and 371 (Table S8). As an internal control, 5S RNA expression was detected on the same Northern blot experiment. Lower panel: quantification of hu mRNA stability in RN6390 (black diamond) and in Δrnc strain (black square) is given as a function of time. The half-life was calculated as described in Figure 5A. The dotted line represents the half-life for the fraction of hu mRNA, which appeared to be degraded in a manner dependent of RNase III. (C) Upper panel: Northern blot analysis of the expression of hu mRNA and the antisense RNA (as-hu) in various strains: RN6390 (wild type strain), Δrnc mutant strain (Δrnc), and the same strain transformed with plasmid expressing the mutant E135A RNase III (Δrnc-E135A), the wild type RNase III (Δrnc-wt), or the mutant D63A RNase III (Δrnc-D63A). A strand specific riboprobe was used to detect as-hu expression. The riboprobe was transcribed in vitro with T7 RNA polymerase using a PCR template amplified with oligonucleotides 270 and 71 (Table S8). Lower panel: Autoradiography showing RNase III cleavage products of 5′ end-labeled hu mRNA alone or associated with the as-hu mRNA. Incubation controls of hu mRNA alone or with hu-as in the absence of RNase III are shown in lanes 1 and 5, respectively. The RNase III cleavage assays were performed in the presence of Mg2+ (lanes 2–4 and 6–8) or Ca2+ (lane 9) on hu mRNA (lanes 2–4) or bound to hu-as (lanes 6–9). The hu mRNA-as-hu duplex was pre-formed with denatured RNAs (denaturing conditions). Increasing concentrations of RNase III were used: 0.165 µM (lanes 2, 6), 0.33 µM (lanes 3, 7) and 0.66 µM (lanes 4, 8, 9). Lanes L, T1: alkaline ladder and RNase T1 ladder of hu mRNA, respectively. The bar denotes the shortest hu mRNA fragments generated by RNase III cleavage upon the as-hu binding.