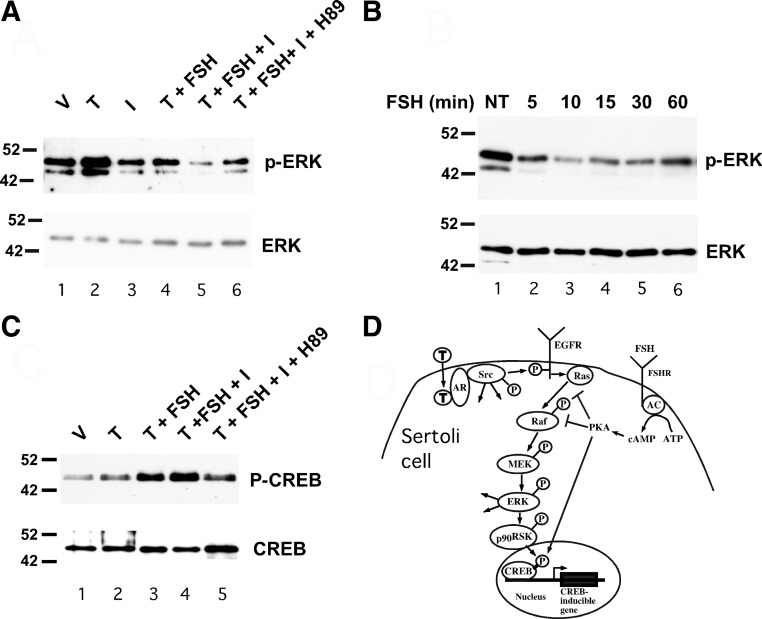
Fig. 1.
FSH inhibits testosterone-mediated activation of ERK via PKA. A, Wild-type Sertoli cells were treated with EtOH-vehicle (V) 100 nm testosterone (T) alone for 10 min or T for 10 min after 20 min pretreatments with various combinations of IBMX (I, 0.5 mm), FSH (100 ng/ml), and H89 (10 μm) as indicated. Whole-cell extracts were assayed by Western blot using antiserum against phosphorylated ERK. The gels were repeated and probed for total ERK. B, Wild-type Sertoli cells were not treated (NT) or treated with FSH for 5–60 min and probed using antisera against phosphorylated ERK and total ERK (A). C, Wild-type Sertoli cells were treated with V, T alone for 10 min, or T for 10 min after 20 min pretreatments with various combinations of FSH (100 ng/ml), IBMX (I, 0.5 mm), and H89 (10 μm) as indicated. Whole-cell extracts were assayed by Western blot using antiserum against CREB phosphorylated at serine 133 or total CREB. The blots shown are representative of at least three independent experiments. D, The nonclassical testosterone signaling pathway initiates with testosterone diffusing through the plasma membrane and binding AR, which recruits and activates Src. The subsequent activation of EGFR, ERK, CREB, and intermediates is shown. Added arrows from Src and ERK represent additional potential activities of these kinases. FSH-mediated increases in adenylate cyclase (AC) activity result in increased conversion of ATP to cAMP, which activates PKA. Activation of PKA inhibits Ras activation of Raf and RAF kinase activity (as demonstrated in Figs. 2 and 3), which blocks phosphorylation and activation of ERK via the MAPK cascade. FSH-mediated activation of PKA also induces CREB phosphorylation and CREB-mediated transcription.