Table 1.
Optimization of template
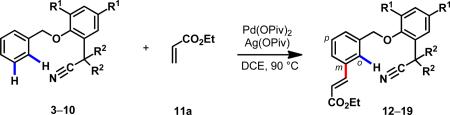
| |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| entry | template | R1 | R2 | yield (%) (mono)b | yield(%) (di)b | selectivity m:p:oc | entry | template | R1 | R2 | yield (%) (mono)b | yield (%) (di)b | selectivity m:p:oc |
| 1d | 3a | tBu | H | trace | 0 | — | 6 | 6 | tBu | —(CH2)4— | 50 | 11 | 88:7:5 |
| 2e,f | 3a | tBu | H | 4 | 0 | 56:26:18 | 7 | 7 | tBu | —(CH2)5— | 51 | 15 | 91:5:4 |
| 3 | 3a | tBu | H | 15 | 0 | 59:33:8 | 8 | 8a | tBu | iBu | 67 | 23 | >95:5(p+o) |
| 4 | 4 | tBu | Me | 60 | 17 | 91:7:2 | 9g | 9 | H | iBu | — | — | — |
| 5 | 5 | tBu | Et | 52 | 16 | 93:6:1 | 10 | 10 | Me | iBu | 39 | 10 | 91:8:1 |
a Unless otherwise noted, the reaction conditions were as follows: benzyl ethers 3–10 (0.05 mmol), olefin 11a (1.5 equiv.), Pd(OPiv)2 (10 mol%), Ag(OPiv) (2.1 equiv.), DCE (0.5 mL), 90 °C, 18 h.
Isolated yield after purification by silica gel column chromatography.
m:p:o denotes the ratio of meta:para:ortho mono-olefinated products, as determined by 1H NMR analysis of the unpurified reaction mixture.
O2 was used as the oxidant instead of Ag(OPiv).
Pd(OAc)2 and Ag(OAc) were used instead of Pd(OPiv)2 and Ag(OPiv) respectively.
NMR conversion was determined by using o-xylene as an internal standard.
C–H olefination occurred on both the arene substrate and on the template with a combined yield of approximately 20% yield.
Abbreviations: DCE, 1,2-dichloroethane; Me, methyl; Et, ethyl; tBu, tert-butyl; OPiv, pivalate.
