Abstract
Purpose.
To determine if glaucoma and/or age-related macular degeneration (AMD) are associated with disability in instrumental activities of daily living (IADLs).
Methods.
Glaucoma subjects (n = 84) with bilateral visual field (VF) loss and AMD subjects (n = 47) with bilateral or severe unilateral visual acuity (VA) loss were compared with 60 subjects with normal vision (controls). Subjects completed a standard IADL disability questionnaire, with disability defined as an inability to perform one or more IADLs unassisted.
Results.
Disability in one or more IADLs was present in 18.3% of controls as compared with 25.0% of glaucoma subjects (P = 0.34) and 44.7% of AMD subjects (P = 0.003). The specific IADL disabilities occurring more frequently in both AMD and glaucoma subjects were preparing meals, grocery shopping, and out-of-home travelling (P < 0.05 for both).
In multivariate logistic regression models run adjusting for age, sex, mental status, comorbidity, and years of education, AMD (odds ratio [OR] = 3.4, P = 0.02) but not glaucoma (OR = 1.4, P = 0.45) was associated with IADL disability. However, among glaucoma and control patients, the odds of IADL disability increased 1.6-fold with every 5 dB of VF loss in the better-seeing eye (P = 0.001). Additionally, severe glaucoma subjects (better-eye MD worse than −13.5 dB) had higher odds of IADL disability (OR = 4.2, P = 0.02). Among AMD and control subjects, every Early Treatment of Diabetic Retinopathy Study line of worse acuity was associated with a greater likelihood of IADL disability (OR = 1.3).
Conclusions.
VA loss in AMD and severe VF loss in glaucoma are associated with self-reported difficulties with IADLs. These limitations become more likely with increasing magnitude of VA or VF loss.
Difficulties with instrumental activities of daily living (IADLs) were evaluated using a structured questionnaire. Patients with more severe levels of glaucoma and age-related macular degeneration were more likely to have IADL disability than controls with normal vision.
Introduction
Given the rapid aging of the population worldwide, the numbers of individuals affected by glaucoma and age-related macular degeneration (AMD) are projected to rise significantly by 2020.1,2 To address the needs of these individuals, it will be crucial to understand how function and quality of life are affected by their disease. Emphasis can then be placed on developing interventions to target specific functional deficits.3
The impact of eye disease on the ability to perform routine daily activities has largely been studied with questionnaires specifically designed to capture the impact of vision loss on vision-specific activities.4 Although such questionnaires suggest lower quality of life with greater vision loss, they are targeted toward visual disability and therefore are not able to frame the impact of vision loss on activities necessary for independent living. The widespread use of vision-specific questionnaires arose largely out of earlier studies that found little or no impact of vision loss on generic quality of life using measures such as the SF-36.5 Possibilities for these findings are that vision does not truly affect independence or that vision-specific questionnaires capture a vision-specific quality of life impairment that is fundamentally dissimilar to the impact of other health conditions.6 However, strong evidence supports the notion that vision loss affects fundamental daily activities required for independent living, such as driving,7,8 reading,9,10 and physical activity.11–13
Two generic measures of disability that have been specifically designed to assess ability to live independently are the activities of daily living (ADL) and instrumental activities of daily living (IADL) questionnaires.14 These assessment tools have been used to demonstrate the disabling effects of a wide range of medical conditions, and this extensive literature provides a useful reference point for understanding the impact of visual disability.15 ADL/IADL questionnaires measure difficulty with basic everyday tasks of fundamental importance, and disability in these tasks comes with great personal and societal costs.16
Several reports have shown associations between vision loss and more frequent ADL/IADL disability,17–20 although most of these studies assessed vision only through self-report of visual ability. The few studies that directly measured vision did not define the cause of vision loss, and evaluated only loss of central acuity, and not visual field (VF) loss.10,21–23 Additionally, findings from these studies were mixed with some studies demonstrating no or weak associations between ADL/IADL disability and vision loss, whereas other studies suggested that visual loss was associated with IADL difficulty, evidenced by performing activities at slower speeds.
Here we assess whether or not patients with visual acuity (VA) loss from AMD or peripheral VF loss from glaucoma are more likely to perform activities of IADL with help or not at all.
Methods
Study Participants
This research was approved by the Johns Hopkins Medical Institutions' Institutional Review Board, and followed the tenets of the Declaration of Helsinki. Written informed consent was obtained from all subjects. Participants were recruited from a convenience sample of patients being cared for at the Glaucoma or Retina Services at the Wilmer Eye Institute at Johns Hopkins. All patients meeting eligibility criteria for the control, glaucoma, or AMD groups were asked about study enrollment on days when research staff were available to discuss study details. Control and glaucoma subjects were enrolled between July 2009 and January 2011, whereas AMD subjects were recruited between January 2010 and June 2011.
Three groups of study participants, controls, glaucoma, and AMD, were recruited. All subjects were aged 60 to 80 and able to communicate in English. Control and glaucoma subjects had completed Humphrey 24-2 VF testing (Carl Zeiss Meditec, Dublin, CA) within 12 months of the study. VA was assessed using the Early Treatment of Diabetic Retinopathy Study (ETDRS) chart. Exclusion criteria for all patients included ocular surgery within the previous 2 months, and/or hospitalizations or procedures within the previous 2 weeks.
Controls were glaucoma suspects or ocular hypertensives with normal VA and VFs. Control subjects had a physician diagnosis of ocular hypertension or glaucoma suspect based on optic nerve and VF findings, and a presenting acuity better than 20/40. VF test results had to show a mean deviation (MD) better than −5 dB in both eyes, with an MD better than −3 dB in at least one eye, and a glaucoma hemifield test (GHT) result other than “outside normal limits” in both eyes.
Glaucoma patients had a physician diagnosis of primary open angle glaucoma, primary angle closure glaucoma, pseudoexfoliation glaucoma, or pigment dispersion glaucoma based on anterior segment, VF, and optic nerve findings. Better-eye VF MD was equal to or worse than −3 dB with a GHT result of “Outside Normal Limits,” “Borderline,” or “Generalized Reduction of Sensitivity” in both eyes. The better-seeing eye was defined as the eye with the higher (less negative) MD.
AMD subjects were required to have bilateral AMD with evidence of drusen, geographic atrophy, or choroidal neovascularization in both eyes. VA was required to be 20/32 or worse in both eyes, or worse than 20/200 in one eye regardless of the second-eye vision.
Assessment of ADLs and IADLs
ADLs and IADLs were assessed using previously described questionnaires.24 Participants were asked to rate each ADL and IADL on a 4-point scale: “No Difficulty,” “With Difficulty, but without help,” “With Help,” or “Unable to Perform Task.” ADLs included the following six activities: walking, bathing or showering, feeding oneself, dressing oneself, using the toilet, and getting out of bed or a chair. IADLs included eight activities: preparing meals, grocery shopping, managing one's own money, using a telephone, heavy housework, light housework, getting to places beyond walking distance, and taking medications. Subjects were considered to have disability regarding a specific ADL or IADL if they reported doing the task with help or not doing the task at all.20,25
Evaluation of Demographic and Health Information
Subjects completed an in-person interview to gather demographic data and information about relevant comorbid conditions. Comorbid conditions were summarized as the number of diseases that individuals self-reported out of a list of 15 different conditions.26 Cognitive ability was assessed through the Mini-mental State Examination (MMSE) for the visually impaired.27 Mood was assessed using the 15-item Geriatric Depression Questionnaire, with a score of 6 or higher indicating the presence of depressive symptoms.28 Height and weight were measured directly and used to calculate body mass index (BMI). Hand grip strength was measured using a Jamar hand dynamometer (Sammons Preston, Inc., Bollingbrook, IL). ETDRS visual acuity was assessed using standard ETDRS charts and converted into logarithm of the minimum angle of resolution (logMAR) score for analysis.29
Statistical Methods
This was a secondary analysis from a study powered to detect differences in physical activity levels between controls and glaucoma and AMD subjects, with intentional oversampling in the glaucoma group.13 In a post hoc sample size analysis, we calculated the detectable group differences using the observed risk of IADL disability in controls, a power of 80% power and a type I error probability of 0.05. Using these parameters, the numbers of recruited patients were sufficient to detect a 2.0- to 2.2-fold difference in any IADL disability among AMD or glaucoma subjects as compared with controls, or a 2.5-fold difference in IADL disability among severe AMD or severe glaucoma subjects as compared with controls. Severe glaucoma subjects were defined as those subjects in the tertile of greatest better-eye VF loss (n = 28), whereas severe AMD subjects were defined as those with a VA at or below the median for AMD subjects (n = 24).
Group differences in the frequency of disability for individual IADL items were assessed using χ2 and Fisher's exact tests to separately compare the AMD and glaucoma groups with the control group. Univariate and multivariate logistic regression were used to test the unadjusted and adjusted associations of demographic, health-related, and visual metrics with IADL disability. Covariates included in the final model were the following: extent of vision loss, age, sex, years of education, number of comorbidities, and MMSE score. Breaking comorbidity out into the impact of specific diseases was explored, but not done given the small numbers of individuals with each disease. Given the different types of vision loss produced by glaucoma and AMD, the impact of glaucoma and VF loss from glaucoma were assessed in separate models, as were the effects of AMD and visual acuity loss from AMD. Final models were assessed using Hosmer-Lemeshow's goodness-of-fit tests. All analyses were performed using Stata 11 (StataCorp LP, College Station, TX).
Results
Sixty control subjects without vision loss, 84 glaucoma patients, and 47 AMD patients completed the study procedures. Neither glaucoma nor AMD subjects differed significantly from control subjects with regard to sex, education level, likelihood of living alone, number of comorbid medical conditions, BMI, grip strength, cognitive ability, or depressive symptoms (P > 0.05 for all) (Table 1). AMD, but not glaucoma, subjects were older than controls (P < 0.001). Glaucoma subjects and controls were more often non-white compared with those with AMD (P < 0.05 for both).
Table 1. .
Characteristics of Mobility Study Participants
|
Controls (n = 60) |
Glaucoma (n = 84) |
AMD (n = 47) |
|
| Demographics | |||
| Age, y (IQR)* | 69.4 (65.2–72.8) | 70.6 (66.4–74.5) | 75.1 (70.9–78.3)† |
| Non-white, % (n) | 23.3 (14) | 39.3 (33)† | 4.3 (2)† |
| Female, % (n) | 61.7 (37) | 53.6 (45) | 57.5 (27) |
| Education, y (IQR) | 16.5 (14–17) | 16 (14–17) | 16 (13–17) |
| Lives alone, % (n) | 18.3 (11) | 19.1 (16) | 21.3 (10) |
| Health | |||
| Comorbidities, n (IQR) | 2 (1–3) | 2 (1–3) | 2(1–3) |
| BMI, kg/m2 (IQR) | 27.9 (23.8–32.2) | 27.7 (24.5–32.0) | 28.3 (24.9–32.7) |
| Grip strength, kg force (IQR) | 26.3 (21.3–32.3) | 28.5 (21.6–36.3) | 26.3 (21.0–33.0) |
| Depressive symptoms, % (n) | 5 (3) | 6 (3) | 4.3 (2) |
| MMSE score (IQR) | 21 (20–22) | 21 (20–22) | 21 (20–22) |
| Vision | |||
| Better-eye MD, dB (IQR) | 0.17 (−0.65:0.9) | −8.0 (−16.7: −4.8)† | — |
| Better-eye VA, logMAR (IQR) | 0.08 (0–0.16) | 0.16 (0.1–0.3)† | 0.40 (0.2–0.7)† |
AMD patients did not have visual field testing.
Median (IQR) reported for continuous variables.
P < 0.05 in comparison of either glaucoma or AMD groups with controls.
Glaucoma subjects had greater VF loss than control subjects with a median better-eye MD of −8.0 dB (interquartile range [IQR] = −16.7 to −4.8 dB) versus a median of 0.2 dB (IQR = −0.6 to +0.1 dB) for control subjects (P ≤ 0.001). Better-eye VA was worse in both glaucoma subjects (median logMAR acuity = 0.16, IQR = 0.08 to 0.33) and AMD subjects (median logMAR acuity = 0.40, IQR = 0.08 to 0.66) as compared with controls (median logMAR = 0.08, IQR = 0.00 to 0.16) (P < 0.001 for both).
Overall, 4 of 191 subjects (2%) described disability in one or more ADLs, whereas 53 of 191 (28%) described disability in one or more IADLs. Of the four subjects with ADL disability, three had glaucoma, one had AMD, and none were controls. Given the small number of participants reporting ADL disability, ADL outcomes were not further analyzed.
Eighteen percent of control subjects had one or more IADL disability, as compared with 25% of glaucoma subjects (P = 0.34), and 44.7% of AMD participants (P = 0.003). Among AMD subjects, the frequency of any IADL disability was 43% in subjects with neovascular AMD in the eye with better VA, and 47% in subjects with non-neovascular AMD in the eye with better VA (P = 0.8). Overall, the three activities with the highest frequency of disability were heavy housework (18.9%), traveling beyond walking distance (14.7%), and grocery shopping (13.6%). Preparing meals, grocery shopping, and traveling outside of the home were more frequently reported by both glaucoma and AMD subjects as compared with controls (P < 0.05 for all). Additionally, subjects with AMD also reported higher rates of disability in managing money, using the phone, heavy housework, and taking medications (P < 0.05 for all) (Table 2). The mean number of IADL disabilities was 0.2 for controls, 0.65 for glaucoma (P = 0.20), and 1.3 for AMD (P < 0.001).
Table 2. .
IADL Disability for Individual Tasks by Disease Status
|
IADL Disablity, % (n) |
Controls (n = 60) |
Glaucoma (n = 84) |
AMD (n = 47) |
| Any IADL Disability | 18.3 (11) | 25 (21) | 44.7 (21)† |
| Individual Task Disability | |||
| Preparing meals | 0 | 7.1 (6)* | 8.5 (4)* |
| Grocery shopping | 1.7 (1) | 15.5 (13)† | 25.5 (12)† |
| Managing money | 0 | 4.8 (4) | 19.2 (9)† |
| Using phone | 0 | 3.6 (3) | 6.4 (3)* |
| Heavy housework | 13.3 (8) | 16.7 (14) | 29.8 (14)* |
| Light housework | 1.7 (1) | 0 | 4.3 (2) |
| Traveling beyond walking distance | 3.33 (2) | 14.3 (12)* | 29.8 (14)† |
| Taking medications | 0 | 3.6 (3) | 8.5 (4)* |
IADL disability defined as needing help with a task or being unable to perform a task even with help.
P < 0.05.
P < 0.01 in unadjusted analyses comparing either the glaucoma or AMD group with controls.
In multivariable models, AMD subjects were more likely to report IADL disability as compared with controls (OR = 3.4, 95% confidence interval [CI] = 1.3 to 9.4, P = 0.02) (Table 3), with slightly higher odds noted for AMD subjects with a better-eye VA of 20/50 or worse (OR = 3.7, 95% CI = 1.1 to 13.0, P = 0.04) (Fig. 1). In multivariable models, glaucoma was not associated with higher odds of IADL disability (P = 0.45), although higher odds were observed in glaucoma subjects in the highest tertile of better-eye VF damage (better-eye MD worse than −13.5 db, OR = 4.2, 95% CI = 1.3 to 13.9, P = 0.02). Among glaucoma and control subjects, a 5 db worsening in better-eye VF MD was associated with a 60% increase in the odds of IADL disability (OR = 1.6, 95% CI = 1.2 to 2.1, P = 0.001) (Fig. 2). Among AMD and control subjects, a one line loss of VA was associated with a 35% increase in the odds of IADL disability (OR = 1.35, 95% CI = 1.1 to 1.6, P < 0.001). Other independent predictors of IADL disability (among all subjects) included less education (OR = 2.0 for 4 years less, 95% CI = 1.1 to 3.7, P = 0.02) and greater comorbid illness (OR = 1.4 per illness, 95% CI = 1.1 to 1.8, P = 0.002). Given the low numbers of non-white AMD participants, the impact of AMD status (OR = 3.0, 95% CI = 1.0 to 9.0, P = 0.045) and severity of VA loss (OR = 1.28 per 1 line decrease in VA, 95% CI = 1.13 to 1.45, P < 0.001) on the likelihood of IADL disability was confirmed to be similar in models in which there were only white control patients.
Table 3. .
IADL Disability by Group and Severity of Vision Loss, Multivariable Analysis
|
Interval |
Group Predictor, OR (95% CI) (n = 191) |
MD Predictor (5 db Worse) (n = 144)* |
Acuity Predictor (1<ETDRS Line) (n = 107)† |
|
| Study Group | ||||
| Glaucoma | vs. Control | 1.4 (0.6–3.6) | — | — |
| AMD | vs. Control | 3.4 (1.3–9.4)‡ | — | — |
| Degree of Damage | ||||
| Better-eye MD* | 5 dB worse | — | 1.6 (1.2–2.1)§ | — |
| Better-eye VA† | 1 < ETDRS line | — | — | 1.3 (1.1–1.6)§ |
| Covariates | ||||
| Age | 5 y older | 1.2 (0.8–1.7) | 1.1 (0.7–1.7) | 1.6 (1.0–2.6) |
| Education | 4 y less | 2.0 (1.1–3.7)‡ | 2.4 (1.1–5.2)‡ | 1.2 (0.5–3.0) |
| Sex | Female vs. male | 1.7 (0.8–3.8) | 3.0 (1.0–9.0)‡ | 2.4 (0.7–7.7) |
| Comorbidity | 1 additional illness | 1.4 (1.1–1.8)§ | 1.6 (1.2–2.2)§ | 1.4 (1.1–2.0)‡ |
| MMSE score | 5 points less | 2.4 (0.8–7.3) | 1.8 (0.4–8.8) | 2.5 (0.5–13.6) |
Model run with control and glaucoma subjects only.
Model run with control and AMD subjects only.
P < 0.05.
P < 0.01.
Figure 1. .
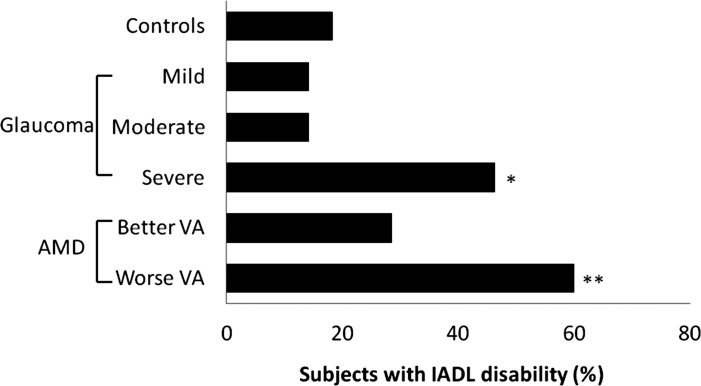
Association of IADL disability with disease severity. Mean better-eye MD was +0.2 dB (IQR = −0.6 to +0.9 dB) in controls (n = 60), −3.8 dB (IQR = −4.8 to −3.2 dB) in the mild glaucoma group (n = 28), −8.0 dB (IQR = −11.1 to −6.9 dB) in the moderate glaucoma group (n = 28), and −19.5 dB (IQR = −16.7 to −25.5 dB) in the severe glaucoma group (n = 28). Mean logMAR better-eye VA was +0.08 (IQR = 0 to +0.16) for controls (n = 60), 0.18 (IQR = 0.1 to 0.28) for the better VA AMD group (n = 23) and 0.65 (IQR = 0.52 to 0.76) for the worse VA AMD group (n = 24). *OR = 4.2 (95% CI 1.3 – 13.9) vs. controls after adjustment for health and demographic covariates. **OR = 3.7 (95% CI 1.1 – 13.0) vs. controls after adjustment for health and demographic covariates.
Figure 2. .
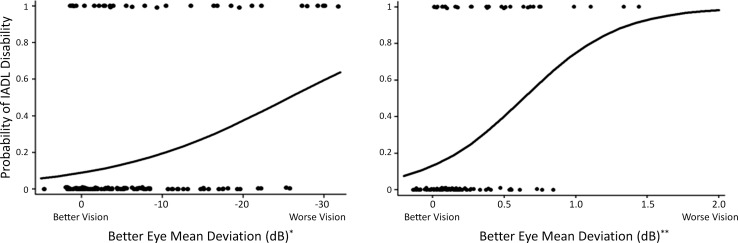
Modeled probability of IADL disability by severity of vision loss. Circles reflect outcomes for individual patients, who either had IADL disability (probability = 1) or did not have IADL disability (probability = 0). Models reflect probability adjusted for age, education, sex, comorbidity, and MMSE score. *Glaucoma and control subjects only. **AMD and control subjects only.
Discussion
The likelihood of reporting one or more IADL limitation increases with severe VF loss from glaucoma and visual acuity loss from AMD. ADL disability, on the other hand, is only rarely affected by VF or VA loss. Although glaucoma itself was not a significant predictor of IADL disability, IADL disability was more common in patients with more severe disease, and increased with greater VF loss, suggesting that differences may not have been observed for the full group due to the lack of an impact on IADLs in patients with mild to moderate VF loss. In AMD subjects, a group effect was seen, although when the group was stratified into better than median vision (20/50) or worse, only those with worse central VA remained significantly affected. This again suggests that IADL disability becomes significantly more likely at more advanced stages of disease.
Impaired mobility may be a primary pathway leading to IADL disability in patients with vision loss. Traveling outside of the home and grocery shopping, in particular, require a means of transportation and the ability to walk in an out-of-home environment with varied terrain. Driving is far and away the primary means of transportation in the United States,30 and driving cessation occurs both with glaucoma and with loss of central visual acuity.7,8 Therefore, individuals with glaucoma and AMD may become dependent on others for these specific tasks as a result of their decision to not drive. Previous research has also demonstrated substantial restriction of walking in glaucoma,7,12,13 and in individuals with bilateral VA loss not due to refractive error.12 These findings are corroborated by a growing body of literature that shows objectively measured mobility restriction in patients with eye disease11,31–33 (also Ramulu PY, Hochberg C, Maul E, et al., unpublished data, 2012). Decreased walking may also result from fear of falling, which may be particularly high outside the home, and which could limit travel outside the home to areas such as the grocery store.
Individuals with glaucoma and AMD also had higher rates of disability in preparing meals (P = 0.04 for both), which may result from a variety of factors. The accuracy of reaching for and grasping objects has been shown to be impaired with both VF and VA loss,34,35 which might cause a fear of dealing with the stove or other hot surfaces. Additionally, cooking requires walking around the kitchen and reading recipes, such that mobility and/or reading impairment may contribute to disability. It is possible that disability with heavy housework among AMD subjects may also result from a combination of fear in handling heavy objects and difficulty with mobility, whereas difficulties with managing money, phone use, and taking medications among AMD subjects likely resulted from difficulty reading.
ADLs were affected at significantly lower rates than the IADL tasks, corroborating the common-sense notion that vision loss (except for extreme cases) may not cause difficulty in the most basic and fundamental tasks of daily life, such as bathing, toileting, and getting dressed. Instead, quality of life may be affected by the need to depend on others for more complex tasks, such as those defined by IADLs. That many glaucoma and AMD subjects did not manifest IADL disability in the current study is likely a result of how IADL disability was defined. Subjects were considered to have disability with an ADL/IADL only if they needed help with the task or were unable to perform the task altogether. It is likely that many more glaucoma and AMD subjects are able to complete these tasks, but only at a slower rate or with more error, as suggested by previous studies that used methods to directly observe task performance.34,36,37 However, the clinical significance of task slowing is often less clear than a total inability to perform the task.
Given the large amount of data regarding ADL/IADL disability in other significant diseases, assessment of the effects of glaucomatous VF loss and AMD-related VA loss with IADLs and ADLs provides a useful framework for comparing the severity of disability from these diseases to other diseases. Our rates of IADL disability were similar to previous studies of IADL disability in vision loss, although most previous studies did not directly evaluate vision.17–20 In data from a 2008 Health and Retirement Survey (HRS)38 of a Canadian population older than 65, 17% of the overall sample reported IADL disability, as compared with 25% of glaucoma subjects and 45% of AMD subjects in our study. These data lend validity to our argument that IADL disability may indeed be more prevalent among individuals with vision loss. In the HRS study, very high rates of IADL disability were noted for older adults with Alzheimer's disease (77%) and stroke (81%), indicating that the disability resulting from vision loss is, on average, not at the same level of severity. On the other hand, several other significant conditions showed comparable rates of IADL disability to the types of vision loss studied here, including back problems (IADL disability in 21% of men and 41% of women), heart disease (24% of men and 48% of women), and urinary incontinence (35% of men and 50% of women). Indeed, in our study, 5 dB more VF loss and 2 additional lines of decreased acuity both had a similar impact on IADL disability compared with the effect of an additional comorbid illness (OR = 1.6 and 1.3 respectively versus 1.4 for comorbid illness).
Several details regarding how the current study was performed limit the generalizability of our findings. For one, because participants were asked to schedule an in-clinic study visit, it is possible that the most-impaired subjects were less likely to join the study, as it would be harder to make an extra trip to the clinic. Indeed, subjects with mild-moderate better-eye VF damage from glaucoma had a slightly lower frequency of IADL disability than controls (although differences were not statistically significant). Study participants generally matched the sex (57% female versus 52% for Maryland), racial (22% African American versus 29% for Maryland), and ethnic distribution (5% Hispanic versus 8% for Maryland) of the local population, but may well have differed from the local population in their health or lifestyle choices. Such differences may have biased our results away from finding an association between eye disease and limitations in ADLs and IADLs. Additionally, glaucoma suspects, and not individuals without eye disease, were chosen as controls. Although these individuals may not be completely normal with regard to their vision, they were felt to be the best control group, as they also had chosen to seek their care at an urban referral center, thereby demonstrating similar health behavior as subjects in the disease groups. Furthermore, these subjects demonstrated a median better-eye VF MD of +0.2 dB and a median VA better than 20/25, suggesting that minimal visual impairment was present. Nonetheless, the choice of glaucoma suspects as controls may have biased our findings toward the null if unmeasured visual difficulties led these individuals to seek care. Findings in certain disease groups, such as all glaucoma subjects, may also have been missed as a result of insufficient study powering. However, much larger samples of patients (568 subjects in each group assuming a power of 0.8 and a type I error probability of 0.05) would be required to detect the observed differences between control and glaucoma. Our relatively small sample size and retrospective design also limited our ability to further stratify our groups into meaningful subsets (e.g., neovascular versus non-neovascular AMD). Last, examining self-reported disability may not reflect true disability in some patients, as self-report can differ from a patient's actual experience/performance.39,40
IADL disability, particularly in mobility-related tasks, is more common in subjects with severe bilateral glaucoma and bilateral VA loss from AMD than in similar individuals with normal sight. Additionally, IADL disability becomes increasingly more likely at greater levels of VF and VA loss. Reading-dependent IADLs are also affected in AMD, but are not more common in glaucoma. Overall, roughly one-half of subjects with severe bilateral glaucoma and severe bilateral AMD have disability in IADLs, indicating that they are not able to perform basic living tasks without the help of another. The frequency of IADL disability among subjects with this degree of vision loss suggests that better patient care can be achieved by the development and implementation of systems to support and rehabilitate the functional losses resulting from VF and VA loss.
Footnotes
Supported by Dennis W. Jahnigen Memorial Award, National Institutes of Health Grant EY018595, the Research to Prevent Blindness Robert and Helen Schaub Special Scholar Award, and the Intramural Research Program of the National Institutes of Health, National Institute of Aging. All funding organizations had no role in the design or conduct of this research.
Disclosure: C. Hochberg, None; E. Maul, None; E.S. Chan, None; S. Van Landingham, None; L. Ferrucci, None; D.S. Friedman, None; P.Y. Ramulu, None
References
- 1. Quigley HA, Broman T. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophtalmol. 2006;90:262–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Friedman DS, O'Colmain BJ, Muñoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophtalmol. 2004;122:564–572 [DOI] [PubMed] [Google Scholar]
- 3. Ramulu PY. Glaucoma and disability: which tasks are affected, and at what stage of disease?. Curr Opin Ophtalmol. 2009;20:92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mangione CM, Lee PP, Gutierrez PR, et al. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophtalmol. 2001;119:1050–1058 [DOI] [PubMed] [Google Scholar]
- 5. Jampel HD, Schwartz A, Pollack I, et al. Glaucoma patients' assessment of their visual function and quality of life. J Glaucoma. 2002;11:154–163 [DOI] [PubMed] [Google Scholar]
- 6. Mangione CM, Phillips RS, Lawrence MG, et al. Improved visual function and attenuation of declines in health-related quality of life after cataract extraction. Arch Ophtalmol. 1994;112:1419–1525 [DOI] [PubMed] [Google Scholar]
- 7. Ramulu PY, West SK, Munoz B, Jampel HD, Friedman DS. Driving cessation and driving limitation in glaucoma: the Salisbury Eye Evaluation Project. Ophthalmology. 2009;116:1846–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Keay L, Munoz B, Turano KA, et al. Visual and cognitive deficits predict stopping or restricting driving: the Salisbury Eye Evaluation Driving Study (SEEDS). Invest Ophthalmol Vis Sci. 2009;50:107–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ramulu PY, West SK, Munoz B, Jampel HD, Friedman DS. Glaucoma and reading speed: the Salisbury Eye Evaluation project. Arch Ophtalmol. 2009;127:82–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. West SK, Rubin GS, Broman AT, et al. How does visual impairment affect performance on tasks of everyday life? The SEE Project. Salisbury Eye Evaluation. Arch Ophtalmol. 2002;120:774–780 [DOI] [PubMed] [Google Scholar]
- 11. Klein BEK, Moss SE, Klein R, Lee KE, Cruickshanks KJ. Associations of visual function with physical outcomes and limitations 5 years later in an older population: the Beaver Dam eye study. Ophthalmology. 2003;110:644–650 [DOI] [PubMed] [Google Scholar]
- 12. Willis J, Jefferys J, Vitale S, Ramulu P. Visual impairment, uncorrected refractive error, and accelerometer-defined physical activity in the United States. Arch Ophtalmol. 2012;130:329–335 [DOI] [PubMed] [Google Scholar]
- 13.Ramulu PY, Maul E, Hochberg C, et al. Real-world assessment of walking and physical activity in glaucoma using an accelerometer. Ophthalmology. [published online ahead of print March 1, 2012]. doi: 10.1016/j.ophtha.2012.01.013. [DOI] [PMC free article] [PubMed]
- 14. Jette AM. Toward a common language for function, disability, and health. Phys Ther. 2006;86:726–734 [PubMed] [Google Scholar]
- 15. Furner SE, Rudberg MA, Cassel CK. Medical conditions differentially affect the development of IADL disability: implications for medical care and research. Gerontologist. 1995;35:444–450 [DOI] [PubMed] [Google Scholar]
- 16. Gustavsson A, Brinck P, Bergvall N, et al. Predictors of costs of care in Alzheimer's disease: a multinational sample of 1222 patients. Alzheimers Dement. 2011;7:318–327 [DOI] [PubMed] [Google Scholar]
- 17. Berger S, Porell F. The association between low vision and function. Journal Aging Health. 2008;20:504–525 [DOI] [PubMed] [Google Scholar]
- 18. Brennan M, Horowitz A, Su Y-P. Dual sensory loss and its impact on everyday competence. Gerontologist. 2005;45:337–346 [DOI] [PubMed] [Google Scholar]
- 19. Laitinen A, Sainio P, Koskinen S, et al. The association between visual acuity and functional limitations: findings from a nationally representative population survey. Ophthalmic Epidemiol. 2007;14:333–342 [DOI] [PubMed] [Google Scholar]
- 20. Griffith L, Raina P, Wu H, Zhu B, Stathokostas L. Population attributable risk for functional disability associated with chronic conditions in Canadian older adults. Age Ageing. 2010;39:738–745 [DOI] [PubMed] [Google Scholar]
- 21. Knudtson MD, Klein BEK, Klein R, Cruickshanks KJ, Lee KE. Age-related eye disease, quality of life, and functional activity. Arch Ophtalmol. 2005;123:807–814 [DOI] [PubMed] [Google Scholar]
- 22. Owsley C, McGwin G, Sloane ME, Stalvey BT, Wells J. Timed instrumental activities of daily living tasks: relationship to visual function in older adults. Optom Vis Sci. 2001;78:350–359 [DOI] [PubMed] [Google Scholar]
- 23. West SK, Munoz B, Rubin GS, et al. Function and visual impairment in a population-based study of older adults The SEE project. Salisbury Eye Evaluation Invest Ophthalmol Vis Sci. 1997;38:72–82 [PubMed] [Google Scholar]
- 24. Shumway-Cook A, Guralnik JM, Phillips CL, et al. Age-associated declines in complex walking task performance: the Walking InCHIANTI toolkit. J Am Geriatr Soc. 2007;55:58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deshpande N, Metter EJ, Lauretani F, et al. Activity restriction induced by fear of falling and objective and subjective measures of physical function: a prospective cohort study. J Am Geriatr Soc. 2008;56:615–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Turano KA, Broman AT, Bandeen-Roche K, et al. Association of visual field loss and mobility performance in older adults: Salisbury Eye Evaluation Study. Optom Vis Sci. 2004;81:298–307 [DOI] [PubMed] [Google Scholar]
- 27. Busse A, Sonntag A, Bischkopf J, Matschinger H, Angermeyer MC. Adaptation of dementia screening for vision-impaired older persons: administration of the Mini-Mental State Examination (MMSE). J Clin Epidemiol. 2002;55:909–915 [DOI] [PubMed] [Google Scholar]
- 28. Friedman B, Heisel MJ, Delavan RL. Psychometric properties of the 15-item geriatric depression scale in functionally impaired, cognitively intact, community-dwelling elderly primary care patients. J Am Geriatr Soc. 2005;53:1570–1576 [DOI] [PubMed] [Google Scholar]
- 29. Bailey IL, Bullimore MA, Raasch TW, Taylor HR. Clinical grading and the effects of scaling. Invest Ophthalmol Vis Sci. 1991;32:422–432 [PubMed] [Google Scholar]
- 30. Eberhard JW. Driving is transportation for most older adults. Geriatrics. 1998;53:S53–S55 [PubMed] [Google Scholar]
- 31. Friedman DS, Freeman E, Munoz B, Jampel HD, West SK. Glaucoma and mobility performance: the Salisbury Eye Evaluation Project. Ophthalmology. 2007;114:2232–2237 [DOI] [PubMed] [Google Scholar]
- 32. Turano KA, Rubin GS, Quigley HA. Mobility performance in glaucoma. Invest Ophthalmol Vis Sci. 1999;40:2803–2809 [PubMed] [Google Scholar]
- 33. Lamoureux EL, Pallant JF, Pesudovs K, et al. Assessing participation in daily living and the effectiveness of rehabilitation in age related macular degeneration patients using the impact of vision impairment scale. Ophthalmic Epidemiol. 2008;15:105–113 [DOI] [PubMed] [Google Scholar]
- 34. Kotecha A, O'Leary N, Melmoth D, Grant S, Crabb DP. The functional consequences of glaucoma for eye-hand coordination. Invest Ophthalmol Vis Sci. 2009;50:203–213 [DOI] [PubMed] [Google Scholar]
- 35. Timberlake GT, Omoscharka E, Quaney BM, Grose SA, Maino JH. Effect of bilateral macular scotomas from age-related macular degeneration on reach-to-grasp hand movement. Invest Ophthalmol Vis Sci. 2011;52:2540–2550 [DOI] [PubMed] [Google Scholar]
- 36. Altangerel U, Spaeth GL, Steinmann WC. Assessment of function related to vision (AFREV). Ophthalmic Epidemiol. 2006;13:67–80 [DOI] [PubMed] [Google Scholar]
- 37. Warrian KJ, Lorenzana LL, Lankaranian D, Dugar J, Wizov SS, Spaeth GL. Assessing age-related macular degeneration with the ADREV performance-based measure. Retina. 2009;29:80–90 [DOI] [PubMed] [Google Scholar]
- 38. Hung WW, Ross JS, Boockvar KS, Siu AL. Recent trends in chronic disease, impairment and disability among older adults in the United States. BMC Geriatr. 2011;11:47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Friedman SM, Munoz B, Rubin GS, et al. Characteristics of discrepancies between self-reported visual function and measured reading speed. Salisbury Eye Evaluation Project Team. Invest Ophthalmol Vis Sci. 1999;40:858–864 [PubMed] [Google Scholar]
- 40. Guralnik JM, Branch LG, Cummings SR, Curb JD. Physical performance measures in aging research. J Gerontol. 1989;44:M141–M146 [DOI] [PubMed] [Google Scholar]