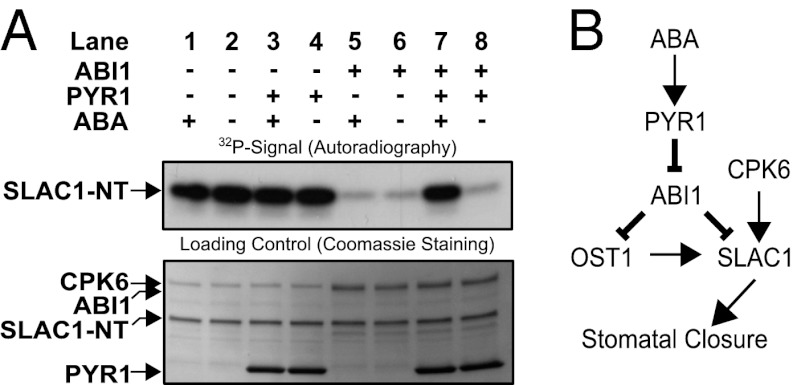
Fig. 6.
(A) In vitro reconstitution of an ABA-dependent signal transduction pathway including PYR1, ABI1, CPK6, and SLAC1-NT protein. Independent of ABA, CPK6 phosphorylates SLAC1-NT (lanes 1 and 2). The presence of ABI1, but not PYR1, inhibits SLAC1-NT phosphorylation, with or without ABA (lanes 3–6). If CPK6, ABI1, and PYR1 are present, addition of ABA leads to the release of ABI1-mediated inhibition of SLAC1 phosphorylation (lane 7). (B) Model for ABA activation of the SLAC1 channel via the OST1 and CPK6 protein kinases and down-regulation by the ABI1 protein phosphatase. ABA binds to PYR1, which causes inhibition of PP2C protein phosphatases, including ABI1. This leads to ABA-induced activation of SLAC1 channels in Xenopus oocytes by CPK6, which functions in native guard cells in ABA activation of S-type anion channels (37). Note that the ABI1 protein phosphatase can directly dephosphorylate the SLAC1 N terminus, which represents a previously unknown target for ABI1 and a mechanism for tight negative SLAC1 regulation, in addition to the known down-regulation of OST1.