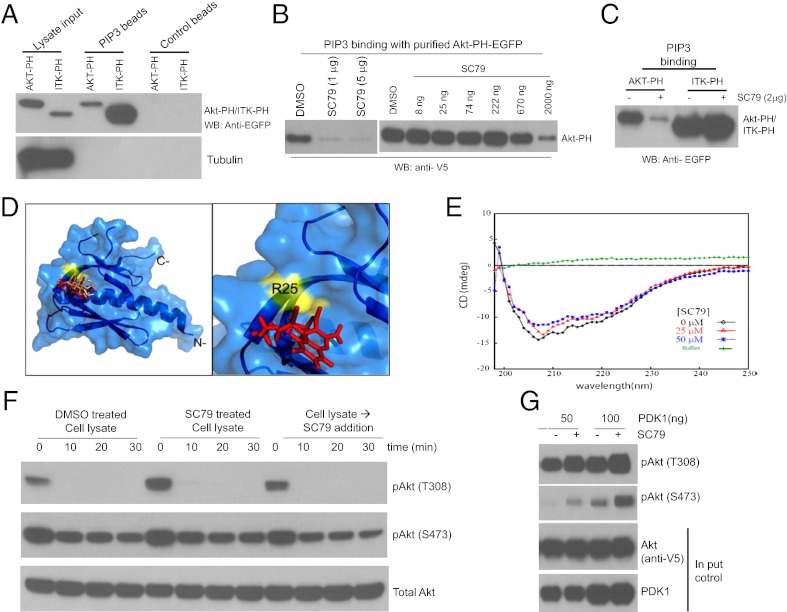
Fig. 3.
SC79 directly binds to Akt and converts it from an inactive conformation to an active conformation, leading to hyperactivation of Akt. (A) Both Akt-PH domain and Itk-PH domain could bind to PtdIns(3,4,5)P3. (B) SC79 inhibited PtdIns(3,4,5)P3 binding function of Akt PH domain. (C) SC79 did not affect the PtdIns(3,4,5)P3 binding function of Itk PH domain. (D) In silico docking of SC79 ligand onto the Akt PH domain structure. (Left) Docked SC79 ligand (rendered red) onto crystal structure of Akt PH domain (PDB ID code: 1UNR, rendered marine). Shown in “wheat” color is the IP4 ligand that docks at the site almost similar to the SC79 binding site. Highlighted in yellow is the residue surface located adjacent to the SC79 binding site that exhibits significant interaction with ligand SC79. (Right) Zoomed region, as in Left, shows docked SC79 ligand and interaction residue R25. (E) CD spectra of human Akt1 and its complex with SC79. An overlay of CD spectra from 5 μM human Akt1 (shown in black) alone and in the presence of SC79 of 25 μM (shown in red) or 50 μM (shown in blue) collected at 25 °C is shown. Baseline was corrected for buffer contribution. (F) SC79 did not affect Akt dephosphorylation in vitro. HEK293 cells grown in serum-rich medium were treated with DMSO or SC79 (4 μg/mL) for 20 min. Cells were lysed on ice in a control buffer devoid of phosphatase inhibitors or supplemented with SC79 (4 μg/mL). After centrifugation at 4 °C, the lysates were kept on ice (time 0 min) or incubated at 37 °C for indicated time points. The kinetics of Akt dephosphorylation at T308 and S473 was determined by Western blot. (G) SC79 directly enhanced Akt phosphorylation by purified recombinant PDK1 in vitro.